Abstract
Background/Aims
The epidemiology of biliary tract cancers has changed in the United States in the past several decades. The aim of this study is to evaluate biliary tract cancers with regard to the incidence rates, etiology, treatment, and survival in Olmsted County between 1976 and 2008.
Methods
Community residents over 20 years of age with a newly diagnosed biliary tract cancers were identified using the Rochester Epidemiology Project. Clinical information, including tumor stage, treatment, and survival status was abstracted from the medical records. The incidence rate was calculated considering the entire population of Olmsted County to be at risk and adjusted by age and sex according to US Census 2000 population. Temporal trends of patient survival with biliary tract cancers were assessed.
Results
A total of 116 subjects met the study criteria. The age-sex-adjusted incidence rate of intrahepatic cholangiocarcinoma(ICC) increased from 0.3 to 2.1 (p=0.02) but one of gall bladder (GB) cancer decreased from 4.0 to 2.2 (p=0.04)per 100,000 person-years between 1976 and 2008 (p<0.01). Overall incidence rates of remaining biliary tract cancers have not changed. Overall 59% of patients presented with stage 3 or 4 cancers and a median survival was 6.3 months. Survival in patients with biliary tract cancer has minimally improved from median survival of 4.2 to 7.7 months between 1976 and 2008 (p=0.05).
Conclusions
In Olmsted County, the incidence of ICC and GB cancer has increased and decreased, respectively. The prognosis remains poor in community residents diagnosed with biliary tract cancers.
Keywords: Etiology, incidence rates, treatment, survival, cause of death
Introduction
Biliary tract cancers, the malignancies involving the biliary drainage system, include cholangiocarcinoma(CCA), ampulla of Vater cancer, and gall bladder (GB) cancer.1 Although it is relatively rare in the US, the prognosis of patients with biliary cancers is extremely poor as they are usually detected at an advanced stage.
The epidemiology of biliary tract cancers has changed in the world including United States over the past three decades. Population based data from the Surveillance Epidemiology and End Results (SEER) program showed an increase in the incidence and mortality rates of intrahepatic cholangiocarcinoma(ICC) in the US.2-4 Similar trends were also observed in the United Kingdom between 1971-2001. In this population, there was a 12-fold increase in the incidence rate of ICC, but a markedly decrease in the incidence of GB and extrahepatic cholangiocarcinoma(ECC).5 Consistent with this trend, there was a global trend of an increasing mortality from ICC and decreasing mortality from ECC.6, 7 However, in contrast, a nation-wide population-based study from Denmark showed a decreasing trend in both ICC and ECC incidence rates.8
In the past two decades, improvements have been made in the treatment of patients with biliary cancers. Innovative interventions, such as liver transplantation following intensive neoadjuvant therapy, have been shown to improve survival if applied in early stages of ECC.9 Further, loco-regional treatment modalities such as radiofrequency ablation, endoscopic/radiographic brachytherapy and systemic chemotherapy may benefit patients with intermediate and advanced stage biliary cancers.10-12 It is unclear, however, whether these new modalities have had any impact on patient outcomes at the population level.
To address these questions, we conducted a population-based study to describe trends in the incidence rates, management, and survival in biliary cancer patients residing in Olmsted County, Minnesota, US between 1976 and 2008.
Method
Database
Epidemiologic research in Olmsted County, Minnesota is possible because the county is relatively isolated from other urban centers and nearly all medical care is delivered to patients in the population by a handful of health care providers. Since 1966, the majority of the health care providers in the community have shared their medical records for research purposes under the auspices of the Rochester Epidemiology Project (REP; R01 AG034676). Currently, the participants in the REP include the Mayo Clinic and its two affiliated hospitals, Olmsted Medical Center and its affiliated hospital, and the Rochester Family Medicine Clinic, the single private practitioner in the County. Together, these providers provide 90-96% of all health care to Olmsted County residents.13 The result is the linkage of medical records from essentially all sources of medical care available to and utilized by the Olmsted County population.
The health care providers who participate in the REP use a unit (or dossier) medical record system whereby all data collected on an individual are assembled in on place.14 These records contain the details of every inpatient hospitalization, every outpatient visit to the offices, clinics, or emergency rooms in the county, every physician visit to nursing homes or private homes, as well as all laboratory results, pathology reports(including autopsies), and correspondence concerning each patient. The medical details are collected by physicians for the delivery of subspecialty level medical care and are of high quality. Additionally, the median duration of medical records is substantial. Virtually everyone in Olmsted County is seen at least once at one of the health care providers in the county within a three-year period. Between 1999 and 2001, 131,879 patients ages 18 and older were seen at a REP provider, representing nearly 100% of the Olmsted County residents in that age range.13, 15 The median number of years encompassed by the combined medical records for Olmsted County residents 18 and older ranged from 12 to 57 years. Therefore, the median years of follow-up available for Olmsted County residents is typically quite high.
Study Population
All Olmsted County residents aged over 20 with a newly diagnosed biliary tract cancers between 1976 and 2008 were identified using the Rochester Epidemiology Project.13, 15 To identify all potential biliary tract cancer patients, Hospital International Classification of Disease Adaptation (HICDA) codes and International Statistical Classification of Diseases and Related Health Problems (ICD)-9 were used. Supplementary table 1 lists all of the codes used to identify potential study subjects. Only local residents who lived within Olmsted County for at least one year prior to cancer diagnosis were included. This was to prevent inclusion of patients who might have moved into Olmsted County for the management of liver disease or biliary tract cancers.
Biliary tract cancers were classified into five disease categories based on the anatomic location of tumor. These include ICC, Perihilar CCA, common bile duct (CBD) CCA, ampulla of Vater cancer, and GB cancer. We defined ICC as a tumor arising from the small intrahepatic bile ductules as a mass-forming lesion in the liver parenchyma beyond the secondary biliary radicals. Perihilar CCA was defined as tumor involving the common hepatic duct, bifurcation of hepatic duct and large intrahepatic duct (up to the secondarybiliary radicals). Diagnosis of biliary tract cancer was made by histology (73%) or characteristics radiographic features (CT or MRI scans or Endoscopic Retrograde Cholangiopancreatography) with (19%) or without (8%) cytology. Characteristics radiographic findings include a tumor arising from the biliary tract or a malignant-appearing biliary stricture with progressive tumor mass on cross-sectional images without evidence of metastasis into biliary tract from other primary sources including the GI tract or pancreas.16 This study was approved by the Institutional Review Board of Mayo Clinic and Olmsted County Medical Center.
Clinical information
Relevant clinical information was abstracted from medical records. These data included risk factors for biliary tract cancers, co-morbidities, tumor staging, primary treatment modality, survival status, and cause of death. For tumor staging, pathologic tumor, node, metastasis (TNM) staging were abstracted if surgical TNM staging was available. If not, clinical TNM staging was recorded based on the cross-sectional images obtained at the time of cancer diagnosis.
Treatment was classified into three groups. The first group included patients who had surgical resection of tumors or liver transplantation. The second group included patients who had systemic or radiation treatment of tumors without surgery. The third group included patients who only had received comfort care.
Vital status of study subjects was assessed as of December 1, 2010. Medical records were reviewed to determine the date of death and identify the cause of death. The cause of death was divided into two categories. A cancer-related death was designated if the patient died of a complication from progressing biliary tract cancer as the main or a contributing condition. Other decedents were classified as cancer-unrelated deaths.
As a part of investigation to understand the changes in biliary tract cancer incidence rates, we considered utilization of cholecystectomy in Olmsted County. All (laparoscopic and open) cholecystectomy procedures performed in Olmsted County residents between 1976-2008 were identified from the REP database. Procedure codes specific for cholecystectomy used in our study included Current Procedural Terminology (CPT) codes (47562, 47563, 47564, 47600, 47605, 47610, 47612, 47620), Berkson codes (2460, 2470, 2480, 2547, 2549), and ICD-9 codes (51.21-51.24).
Statistical analysis
The R (http://www.r-project.org/) and SAS (SAS Institute, Cary, NC) packages were used for statistical analyses. In calculating incidence rates in the community, the entire population of Olmsted County (age ≥20) was considered to be at risk. Age-specific person–years were estimated from decennial census data with linear interpolation between census years. With the assumption that incident cases follow a Poisson distribution, 95% confidence intervals for incidence rate were calculated. Incidence rate was adjusted to the population structure of the United States in 2000. Trend for incidence rates were tested by fitting a Poisson regression model where cancer counts was the outcome and age, sex, and era were independent predictors. The model was adjusted for the population size of each era.
Patient survival was assessed from the time of cancer diagnosis to the last known follow-up or death. In patients who have not died or were lost to follow-up, the observation was censored on the day of last follow-up. Survival probabilities were estimated using the Kaplan-Meier method and compared by the log rank test.
Result
Patient characteristics
Initially, 216 subjects with a diagnosis of biliary tract cancers were identified in the REP database. Upon review of the records, 77 patients were excluded because patients did not have primary biliary tract cancers: these include pancreatic cancer (n=27), cancer of other known primary (n=22), cancer of unknown origin (n=5), hepatocellular carcinoma (n=2) and other benign diagnoses (n=21). Eighteen patients were excluded because the 1-year residency requirement was not met and 5 patients were excluded because cancer diagnosis was made prior to 1976. The remaining 116 patients were included in this analysis.
Table 1 summarizes the baseline characteristics of patients in this study. The mean age of our cohort was 71 years and half were male. There was a female preponderance in patients with GB cancers and most patients were white. A quarter of patients with ICC and a smaller proportion of patients with perihilar CCA (14%) also had evidence of cirrhosis. PSC was present only in a small subset of patients with perihilar CCA (7%). Only 2% had family history of biliary tract cancers in a first degree relative. As expected, the majority of patients with gall bladder cancers had a history of gall stone disease while only 5% had porcelain GB. No patients in our cohort had evidence of chronic viral hepatitis, hepatolithiasis, biliary tract anomaly or parasite infection.
Table 1. Characteristics of Olmsted County Residents Diagnosed with Biliary Tract Cancers.
| ICC (n=16) | Perihilar CCA (n=28) | CBD CCA (n=16) | Ampulla of Vater (n=14) | GB cancer (n=42) | Overall (n=116) | P value | |
|---|---|---|---|---|---|---|---|
| Age | 69±13 | 68±14 | 72±12 | 72±12 | 72±12 | 71±12 | 0.66 |
| Male | 10 (63%) | 14 (50%) | 9 (56%) | 7 (50%) | 13 (31%) | 53 (46%) | 0.16 |
| Race | |||||||
| White | 16 (100%) | 28 (100%) | 15 (94%) | 14 (100%) | 41 (98%) | 114 (98%) | 0.69 |
| Diagnosis | 0.10 | ||||||
| Histology | 8 (50%) | 18 (64%) | 13(81%) | 13 (93%) | 33 (79%) | 85 (73%) | |
| Cytology | 7 (44%) | 6 (22%) | 1 (6%) | 1 (7%) | 7 (17%) | 22 (19%) | |
| Radiology | 1 (6%) | 4 (14%) | 2 (13%) | 0 (0%) | 2 (4%) | 9 (8%) | |
| Risk factors | |||||||
| Liver Cirrhosis | 4 (25%) | 4 (14%) | 0 (0%) | 0 (0%) | 0 (0%) | 8 (7%) | <0.01 |
| PSC | 0 (0%) | 2 (7%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (2%) | 0.21 |
| Alcohol | 2 (13%) | 3 (11%) | 1 (6%) | 1 (7%) | 3 (7%) | 10 (9%) | 0.94 |
| Smoking | 11 (83%) | 20 (71%) | 11 (73%) | 7 (50%) | 21(50%) | 70 (62%) | 0.23 |
| Family history | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (5%) | 2 (2%) | 0.83 |
| Gall stone | 4 (25%) | 10 (36%) | 5 (31%) | 4 (29%) | 34(81%) | 57 (49%) | <0.01 |
| Porcelain GB | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (5%) | 2 (2%) | 0.82 |
ICC, intrahepatic cholangiocarcinoma; CCA, cholangiocarcinoma; CBD, common bile duct; GB, gall bladder; PSC, primary sclerosing cholangitis
Incidence rates of biliary cancers
Table 2 shows the temporal trends in age-adjusted incidence rates for biliary tract cancers in Olmsted County between 1976 and 2008. The overall age-sex-adjusted incidence rate of ICC increased from 0.3 to 2.1 per 100,000 person-years between 1976 and 2008(p=0.02). On the other hand, the overall age-sex-adjusted incidence rate of gall bladder cancer decreased from 4.0 to 2.2 per 100,000 person-years between 1976 and 2008 (p=0.04). The increase in the incidence of ICC was predominantly seen in men, whereas the decrease in the incidence of GB cancer was mainly in women. The overall incidence rates of biliary tract cancers showed no significant change over time (p=0.81).
Table 2. Incidence rates for Biliary Tract Cancers in Olmsted County Residents.
| 1976-1990 (n=46) | 1991-2000 (n=29) | 2001-2008 (n=41) | P value | ||
|---|---|---|---|---|---|
| ICC | Overall | 0.3 (0-0.8) | 0.8 (0-1.5) | 2.1 (0.8-3.4) | 0.02 |
| Men | 0.4 (0-1.1) | 0.9 (0-2.2) | 3.6 (0.9-6.3) | 0.03 | |
| Woman | 0.3 (0-0.9) | 0.8 (0-1.8) | 1.1 (0-2.3) | 0.29 | |
| Perihilar CCA | Overall | 2.2 (0.9-3.4) | 1.9 (0.6-3.1) | 1.4 (0.4-2.4) | 0.32 |
| Men | 2.3 (0.4-4.1) | 2.9 (0.5-5.3) | 1.0 (0-2.5) | 0.23 | |
| Woman | 1.9 (0.4-3.4) | 1.2 (0-2.5) | 1.8 (0.2-3.4) | 0.85 | |
| CBD CCA | Overall | 1.3 (0.2-2.3) | 0.6 (0-1.3) | 1.5 (0.4-2.6) | 0.68 |
| Men | 2.3 (0-4.7) | 0 (0-0) | 2.7 (0.3-5.1) | 0.64 | |
| Woman | 0.7 (0-1.7) | 1.2 (0-2.5) | 0.5 (0-1.3) | 0.92 | |
| Ampulla of Vater cancer | Overall | 0.7 (0-1.5) | 0.6 (0-1.4) | 1.5 (0.4-2.6) | 0.28 |
| Men | 1.1 (0-2.7) | 0.4 (0-1.2) | 1.8 (0-3.6) | 0.34 | |
| Woman | 0.6 (0-1.4) | 0.8 (0-1.8) | 1.1 (0-2.5) | 0.58 | |
| GB cancer | Overall | 4.0 (2.3-5.7) | 2.1 (0.8-3.3) | 2.2 (0.8-3.5) | 0.04 |
| Men | 2.6 (0.2-5.0) | 1.0 (0-2.3) | 3.2 (0.6-5.8) | 0.68 | |
| Woman | 5.0 (2.6-7.5) | 2.8 (0.8-4.8) | 1.6 (0-3.2) | 0.01 | |
| Biliary tract cancer | Overall | 8.5 (6.0-11.0) | 6.0 (3.8-8.2) | 8.6 (6.0-11.3) | 0.81 |
| Men | 8.8 (4.5-13.0) | 5.2 (2.1-8.3) | 12.3 (7.3-17.3) | 0.25 | |
| Woman | 8.5 (5.3-11.7) | 6.7 (3.6-9.8) | 6.1 (3.1-9.1) | 0.17 |
Test for trend over time
ICC, intrahepatic cholangiocarcinoma; CCA, cholangiocarcinoma; CBD, common bile duct; GB, gall bladder
A sensitivity analysis was performed after excluding patients without histologic or cytologic confirmation of diagnosis. The change in the incidence rates of ICC remained significant. However it was no longer significant in GB cancers: In the first (1976-1990), second (1991-2000), and third (2001-2008)era, the overall age-sex-adjusted incidence rate of ICC was 0.3 (95% CI, 0-0.8), 0.8 (95% CI, 0-1.5), and 2.0 (95% CI, 0.7-3.3)per 100,000 person-years (p=0.03). On the other hand, the overall age-sex-adjusted incidence rate of gall bladder cancer was 3.7 (95% CI, 2.1-5.3), 2.1 (95% CI, 0.8-3.3), and 2.2 (95% CI, 0.8-3.5)per 100,000 person-years (p=0.09).
As there were significant changes in the incidence rate of ICC and GB cancer, we investigated the potential underlying cause of this trend. We first examined the proportion of patients with cirrhosis among ICC patients as cirrhosis has been proposed as a risk factor for ICC. Neither of the two ICC patients in the first era (0%) had cirrhosis, whereas one out of the four ICC patients in the second era (25%), and three out of ten in the third era (30%) had evidence of cirrhosis. Next, in relation to the decreased incidence of GB cancer, we calculated the incidence rates of cholecystectomy in the Olmsted county community. The age- and sex-adjusted incidence rate of cholecystecomy in Olmsted County increased over the study period. This increasing trend was statistically significant in women.(Table 3)
Table 3. Incidence rates of Cholecystectomy in Olmsted County Residents.
| 1976-1990 (n=2652) | 1991-2000 (n=2198) | 2001-2008 (n=2609) | P value | ||
|---|---|---|---|---|---|
| Cholecystectomy | Overall | 215 (207-224) | 202 (193-210) | 251 (241-261) | <0.01 |
| Men | 176 (163-188) | 128 (118-139) | 156 (144-167) | 0.64 | |
| Woman | 258 (246-271) | 276 (263-290) | 347 (331-363) | <0.01 |
Clinical Management and Outcome of Biliary Cancers
Initial tumor staging, treatment approaches, and patient survival in individual cancers are described in Table 4. Overall, 59% of patients had TNM stage 3 or 4 biliary tract cancers at initial presentation. While the majority of patients with GB cancer (90%) had stage 3 or 4 disease, only 23% of patients with CBD or ampulla of Vater cancer had stage 3 or 4 disease. Consequently, patients with CBD or ampulla of Vater cancer were more likely to receive surgical treatment.
Table 4. Tumor Stage, Primary Treatment, Survival, and Cause of Death for Biliary Tract Cancer Patients.
| ICC (n=16) | Perihilar CCA (n=28) | CBD CCA (n=16) | Ampulla of Vater (n=14) | GB cancer (n=42) | Overall (n=116) | P value | |
|---|---|---|---|---|---|---|---|
| TNM | <0.01 | ||||||
| 1-2 | 6 (37%) | 13 (50%) | 13 (81%) | 10 (77%) | 4 (9%) | 46 (41%) | |
| 3-4 | 10 (63%) | 13 (50%) | 3 (19%) | 3 (23%) | 38 (91%) | 67 (59%) | |
| Treatment | 0.03 | ||||||
| Surgical treatment* | 5 (32%) | 8 (29%) | 8 (54%) | 10 (72%) | 24 (58%) | 55 (48%) | |
| Systemic or radiation treatment | 6 (38%) | 4 (14%) | 2 (13%) | 0 (0%) | 4 (10%) | 16 (14%) | |
| Comfort care | 5 (31%) | 16 (57%) | 5 (33%) | 4 (29%) | 14 (34%) | 44 (38%) | |
| Survival | 0.88 | ||||||
| Median (month) | 4.1 | 7.2 | 10.2 | 11.1 | 3.8 | 6.3 | |
| 1 year | 38% | 32% | 44% | 50% | 24% | 34% | |
| 5 year | 13% | 6% | 6% | 9% | 14% | 10% | |
| Death | 14 (88%) | 26 (93%) | 16 (100%) | 12 (86%) | 38 (90%) | 106 (91%) | 0.61 |
| Death from Biliary Tract cancer | 14 (100%) | 26 (100%) | 15 (94%) | 8 (67%) | 35 (92%) | 98 (92%) | 0.07 |
One ICC patient who was initially misdiagnosed with HCC and two perihilar CCA patients received liver transplantation. ICC, intrahepatic cholangiocarcinoma; CCA, cholangiocarcinoma; CBD, common bile duct; GB, gall bladder
As a result of a high proportion of tumors detected at an advanced stage, the overall median survival in patients with biliary tract cancer was only 6.3 months, with a 5-year survival of mere 6-14%. Over time, there was minimal improvement in the overall survival (4.2 to 7.7 months between 1976 and 2008, Figure 1). After adjusting for the location of tumors, this change in the overall survival was marginally significant (p=0.07).Patients who received curative surgical treatment had better survival – their median survival was 50 months whereas that for those who did not undergo curative resection had was only 3.8 months (P<0.01). This trend was observed in each subtype of cancers. (supplementary table 2).
Figure 1.
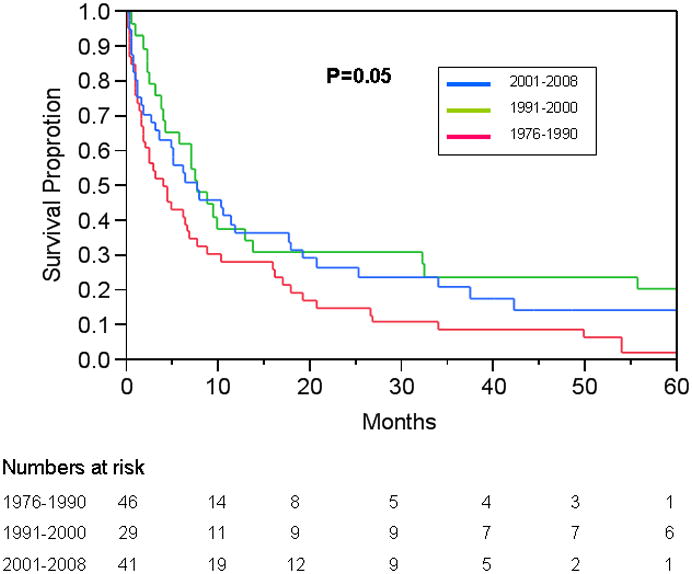
Trends in the survival of Olmsted County, Minnesota residents with Biliary Tract Cancer 1976-2008. Figure footnote: The change in survival became significant (p=0.02) when the latter two eras were combined – perhaps reflecting advances (e.g., portal vein embolization) in surgical management in the early 1990s.25
With regard to the cause of death, while most patients died of their biliary tract cancer, a third of patients with ampulla of Vater cancer died of causes other than the cancer. When only cancer-related death was considered , survival in patients with ampulla of Vater cancer was longer, with median survival of 23, compared to 6.0 months in patients with other subtypes ( P<0.05).
Discussion
In this population-based study investigating the trend in the incidence rate of biliary tract cancers in Olmsted County, the incidence of ICC has increased and the incidence of GB cancer has decreased over the past three decades. When these malignancies were grouped together, the overall incidence did not change. The majority of patients presented with advanced stage cancers, leading to a poor overall survival, although there was a trend for better survival in more recent patients.
Several studies to date have shown that the incidence of ICC has increased in the US.2-4 These studies were performed using the SEER data. Patel first described the increasing incidence of ICC in the US (ICD-9-CM [Clinical Modification] code=155.1).2 Age specific incidence rates of ICC increased from 0.1 per 100,000 in 1973 to 0.7 per 100,000 person-years in 1997. A second study using the same data (ICD-O=8160, 8162, 8260, 8481, 8500, 8560) reported that the incidence of ICC increased from 0.3 to 0.9 per 100,000 person year between 1975-19993. In that study, 7% of ICC cases were ‘Klatskin’ tumors, which were misclassified as ICCs. In another study by Welzel et al, Klatskin tumors were reclassified as ECCs but the rise in the incidence persisted (0.3 to 0.8 per 100,000 person year between 1973-2001).4 Despite methodological differences, these studies consistently documented a rise in the incidence rates of ICC consistently, which were similar to the incidence rates generated in this study. Further, our data extend the observation that the incidence of ICC continued to increase even after 2000.
The reason for the increasing incidence of ICC remains to be completely understood. Although small in number, our data point to the possibility that an increasing number of ICC patients harbor cirrhosis in the background. Although the majority of our patients did not have viral hepatitis or cirrhosis, our data are congruent with other investigators who have suggested that chronic liver disease and cirrhosis constitute an important risk factor for ICC.17
Similar to ICC, studies investigating the incidence of GB cancers were performed using the SEER data. Garriaga et al first reported that the incidences of GB cancer had slightly decreased from 1.4 to1.2 per 100,000 person years between 1973-1987.18 More recent studies also confirmed the decreasing incidence of GB cancer between 1973 and 2002.19, 20 Thus, the decreasing incidence rates of GB cancer in Olmsted County is confirmatory to the previously reported national trend. However, the absolute rate of GB cancer incidence (2.2 to 4.0 per 100,000 person years) was much higher in Olmsted County than the incidence rates reported based on the SEER data (1.1 to 1.8 per 100,000 person years). Exclusion of GB cancers that were not histologically confirmed in previous studies might have, in part, underestimated the incidence of GB cancers.18
Our data showed that the reduction of GB cancer incidence in Olmsted County was mainly driven by the trend in women while it did not change materially in men. While this needs further confirmation in another population, the reciprocal increase in the utilization rate of cholecystectomy during the same period suggests that the removal of diseased gallbladders may in fact reduce the number at risk for GB cancer. There have been other studies that suggested that adoption of laparoscopic cholecystectomy may have contributed to the trend. 21, 22 Clearly, however, one should not construe these data to indicate that prophylactic cholecystectomy in an asymptomatic patient with gallstones may be warranted from the standpoint of reducing the future risk of GB cancer as only small portion of patient develop GB cancer and risk and cost related to surgery does not does not exceed the potential benefit.23, 24
Long term survival was extremely low in patients with biliary tract cancers although there was a trend of improvement in 1 year and 5 year survival over the past 3 decades in Olmsted County. Trend in the overall survival after the diagnosis of biliary tract cancers was previously reported in SEER studies. For example, the 1-year survival for ICCs slightly improved from 16% to 28% between 1975 to 1999. 3 Similarly, the median survival in patients with GB cancer increased from 4 to 6 months between 1973 and 2002.19 We believe that survival improvement in patients with biliary tract cancer is in part attributed to advances in surgical technique as well as improvement in non surgical modalities, such as chemotherapy and radiation treatment.25 Despite these increase in survival in patients with ICC and GB cancers, the overall survival of all bile duct cancers has not changed with a five-year survival of 10% between 1979 and 2004.20 Consistent with national statistics, our results suggest that survival of patients with biliary tract cancer remained very poor despite minimal improvement over the past three decades. Consistent with previous study, our data showed that the prognosis of patients with Ampulla of Vater cancer is better than in patients with other subtypes of biliary tract cancer.18 The cancer detection at an earlier stage, thus higher proportion of patient receiving surgical treatment may explains this better survival in patients with Ampulla of Vater.
There are several weaknesses in our study. First, data from Olmsted County may not be generalizable to the entire US in terms of racial/ethnic distribution, disease patterns and access to care. Individuals of Caucasian race, known to have lower incidence of biliary tract cancers, is over-represented in Olmsted County,20 which will tend to underestimate the true incidence rates of biliary tract cancers in the US. In terms of the access to care, although local patients had better access to specialized care (e.g., Mayo Clinic) than most communities, the poor survival result in this study indicates it provided no real advantage. Second, our study is based on a relatively small number of cases due to the small size of the local population. Therefore, we refrained from performing detailed trend analyses within each subset of biliary tract cancers. This limitation in the sample size must be gauged against the advantages of the study setting in Olmsted County where we were able to identify all biliary cancer patients, to accurately enumerate the denominator population, and to access medical records in the community. This last point is a substantial strength of this study, as studies based on diagnostic codes may fail to accurately discern the location of the tumor - a third of CCA in the SEER database does not have a location specified 20.
In summary, the incidence of ICC in Olmsted County increased and the incidence of GB cancer decreased over the past three decades. The overall incidence rate of biliary tract cancer has not changed. Although there was minimal improvement in survival, prognosis of patients with biliary tract cancers remains extremely poor. To the extent that these epidemiologic trends have been reproducible, urgent efforts are needed to elucidate reasons for these observations.
Study Highlights.
What is Current Knowledge
Biliary tract cancers are rare but fatal diseases.
The trend of epidemiology and clinical outcome in patients with biliary tract cancers is poorly documented.
What is New Here
The incidence of intrahepatic cholangiocarcinoma increased over the past three decades.
The incidence of gall bladder cancer decreased, notably in women.
The increasing trend of cholecystectomy in the community may explain the decreasing incidence trend of gall bladder cancer.
The clinical outcome of biliary tract cancer has improved, albeit minimally.
Acknowledgments
Financial support: This study was funded by a grant (DK-84832; DK-34238) from the National Institute of Diabetes, Digestive and Kidney Diseases, Rochester Epidemiology Project (Grant #R01- AG034676 from the National Institute on Aging), and Clinical and Translational Science Awards (Grant #UL1- RR024150 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health. These fundings were independent of the current study design, collection, analysis and interpretation.
Guarantor of the article: W. Ray Kim, M.D.
Abbreviations
- CCA
cholangiocarcinoma
- ICC
intrahepaticcholangiocarcinoma
- ECC
extrahepaticcholangiocarcinoma
- GB
gallbladder
Footnotes
Disclosure: No conflict of interest
Potential competing interests: None
Specific author contributions: Ju Dong Yang contributed to study design, acquisition of data, analysis and interpretation of data, and drafting of the manuscript; Bohyun Kim, Schuyler O. Sanderson, contributed to study concept and design, interpretation of data, and critical revision of the manuscript; Joseph J. Larson and contributed to analysis and interpretation of data; Terry M. Therneau contributed to analysis and interpretation of data, critical revision of the manuscript for important intellectual content; Jennifer St. Sauver, Barbara P. Yawn,Gregory J. Gores, and Lewis R. Roberts contributed to study concept and design, interpretation of data, and critical revision of the manuscript; W. Ray Kim contributed to study concept and design, interpretation of data, study supervision, obtaining funding, and critical revision of the manuscript. All authors approved final draft submitted.
References
- 1.de Groen PC, Gores GJ, LaRusso NF, Gunderson LL, Nagorney DM. Biliary tract cancers. N Engl J Med. 1999;341:1368–78. doi: 10.1056/NEJM199910283411807. [DOI] [PubMed] [Google Scholar]
- 2.Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology. 2001;33:1353–7. doi: 10.1053/jhep.2001.25087. [DOI] [PubMed] [Google Scholar]
- 3.Shaib YH, Davila JA, McGlynn K, El-Serag HB. Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase? J Hepatol. 2004;40:472–7. doi: 10.1016/j.jhep.2003.11.030. [DOI] [PubMed] [Google Scholar]
- 4.Welzel TM, McGlynn KA, Hsing AW, O'Brien TR, Pfeiffer RM. Impact of classification of hilar cholangiocarcinomas (Klatskin tumors) on the incidence of intra- and extrahepatic cholangiocarcinoma in the United States. J Natl Cancer Inst. 2006;98:873–5. doi: 10.1093/jnci/djj234. [DOI] [PubMed] [Google Scholar]
- 5.West J, Wood H, Logan RF, Quinn M, Aithal GP. Trends in the incidence of primary liver and biliary tract cancers in England and Wales 1971-2001. Br J Cancer. 2006;94:1751–8. doi: 10.1038/sj.bjc.6603127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khan SA, Taylor-Robinson SD, Toledano MB, Beck A, Elliott P, Thomas HC. Changing international trends in mortality rates for liver, biliary and pancreatic tumours. J Hepatol. 2002;37:806–13. doi: 10.1016/s0168-8278(02)00297-0. [DOI] [PubMed] [Google Scholar]
- 7.Patel T. Worldwide trends in mortality from biliary tract malignancies. BMC Cancer. 2002;2:10. doi: 10.1186/1471-2407-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jepsen P, Vilstrup H, Tarone RE, Friis S, Sorensen HT. Incidence rates of intra- and extrahepatic cholangiocarcinomas in Denmark from 1978 through 2002. J Natl Cancer Inst. 2007;99:895–7. doi: 10.1093/jnci/djk201. [DOI] [PubMed] [Google Scholar]
- 9.Rosen CB, Heimbach JK, Gores GJ. Surgery for cholangiocarcinoma: the role of liver transplantation. HPB (Oxford) 2008;10:186–9. doi: 10.1080/13651820801992542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gusani NJ, Balaa FK, Steel JL, Geller DA, Marsh JW, Zajko AB, Carr BI, Gamblin TC. Treatment of unresectable cholangiocarcinoma with gemcitabine-based transcatheter arterial chemoembolization (TACE): a single-institution experience. J Gastrointest Surg. 2008;12:129–37. doi: 10.1007/s11605-007-0312-y. [DOI] [PubMed] [Google Scholar]
- 11.Park SY, Kim JH, Yoon HJ, Lee IS, Yoon HK, Kim KP. Transarterial chemoembolization versus supportive therapy in the palliative treatment of unresectable intrahepatic cholangiocarcinoma. Clin Radiol. 2011;66:322–8. doi: 10.1016/j.crad.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira SP, Roughton M, Bridgewater J. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–81. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 13.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–74. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 14.Connolly DC, Oxman HA, Nobrega FT, Kurland LT, Kennedy MA, Elveback LR. Coronary heart disease in residents of Rochester, Minnesota, 1950-1975. I. Background and study design. Mayo Clin Proc. 1981;56:661–4. [PubMed] [Google Scholar]
- 15.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ, 3rd, Rocca WA. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester epidemiology project. Am J Epidemiol. 2011;173:1059–68. doi: 10.1093/aje/kwq482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moreno Luna LE, Kipp B, Halling KC, Sebo TJ, Kremers WK, Roberts LR, Barr Fritcher EG, Levy MJ, Gores GJ. Advanced cytologic techniques for the detection of malignant pancreatobiliary strictures. Gastroenterology. 2006;131:1064–72. doi: 10.1053/j.gastro.2006.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shaib YH, El-Serag HB, Davila JA, Morgan R, McGlynn KA. Risk factors of intrahepatic cholangiocarcinoma in the United States: a case-control study. Gastroenterology. 2005;128:620–6. doi: 10.1053/j.gastro.2004.12.048. [DOI] [PubMed] [Google Scholar]
- 18.Carriaga MT, Henson DE. Liver, gallbladder, extrahepatic bile ducts, and pancreas. Cancer. 1995;75:171–90. doi: 10.1002/1097-0142(19950101)75:1+<171::aid-cncr2820751306>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 19.Kiran RP, Pokala N, Dudrick SJ. Incidence pattern and survival for gallbladder cancer over three decades--an analysis of 10301 patients. Ann Surg Oncol. 2007;14:827–32. doi: 10.1245/s10434-006-9224-4. [DOI] [PubMed] [Google Scholar]
- 20.Everhart JE, Ruhl CE. Burden of digestive diseases in the United States Part III: Liver, biliary tract, and pancreas. Gastroenterology. 2009;136:1134–44. doi: 10.1053/j.gastro.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 21.Diehl AK, Beral V. Cholecystectomy and changing mortality from gallbladder cancer. Lancet. 1981;2:187–9. doi: 10.1016/s0140-6736(81)90366-4. [DOI] [PubMed] [Google Scholar]
- 22.Wood R, Fraser LA, Brewster DH, Garden OJ. Epidemiology of gallbladder cancer and trends in cholecystectomy rates in Scotland, 1968-1998. Eur J Cancer. 2003;39:2080–6. doi: 10.1016/s0959-8049(03)00370-8. [DOI] [PubMed] [Google Scholar]
- 23.Maringhini A, Moreau JA, Melton LJ, 3rd, Hench VS, Zinsmeister AR, DiMagno EP. Gallstones, gallbladder cancer, and other gastrointestinal malignancies. An epidemiologic study in Rochester, Minnesota. Ann Intern Med. 1987;107:30–5. doi: 10.7326/0003-4819-107-1-30. [DOI] [PubMed] [Google Scholar]
- 24.Ransohoff DF, Gracie WA, Wolfenson LB, Neuhauser D. Prophylactic cholecystectomy or expectant management for silent gallstones. A decision analysis to assess survival. Ann Intern Med. 1983;99:199–204. doi: 10.7326/0003-4819-99-2-199. [DOI] [PubMed] [Google Scholar]
- 25.Abdalla EK, Hicks ME, Vauthey JN. Portal vein embolization: rationale, technique and future prospects. Br J Surg. 2001;88:165–75. doi: 10.1046/j.1365-2168.2001.01658.x. [DOI] [PubMed] [Google Scholar]


