Abstract
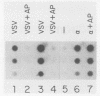
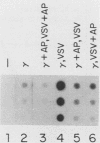
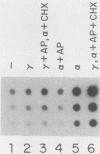
Transcription of several interferon-inducible human genes is also induced by double-stranded RNA. The nature and the mechanism of action of signals generated by interferons and by double-stranded RNA which mediate the induction of these genes are under investigation. Here we report that 2-aminopurine, a known inhibitor of protein kinases, could selectively block this induction process. Induction of mRNAs 561 and 6-16 in HeLaM cells by double-stranded RNA was completely inhibited by 10 mM 2-aminopurine, whereas cellular protein and RNA syntheses as well as the induction of metallothionein mRNA by CdCl2 were unaffected by this inhibitor. In addition, 2-aminopurine blocked the induction of the same two mRNAs and of mRNAs 2-5(A) synthetase, 2A, and 1-8 by alpha interferon and of mRNAs 2A and 1-8 by gamma interferon in HeLaM cells. The observed inhibition was at the level of transcription, and for establishing efficient inhibition, the 2-aminopurine treatment had to begin at early stages of interferon treatment. In GM2767 cells, 2-aminopurine inhibited induction of mRNAs 561 and 6-16 by double-stranded RNA but not by alpha interferon. These results suggest that double-stranded RNA-induced signal 2 is distinct from the interferon-alpha-induced signal 2 (R. K. Tiwari, J. Kusari, and G. C. Sen, EMBO J. 6:3373-3378, 1987) and that 2-aminopurine can block the former but not the latter. Moreover, it appeared that 2-aminopurine could block the production of signal 1 by interferons. This was confirmed by experiments in which we separately tested the effects of 2-aminopurine on signal 1 and signal 2 production by interferons in HeLaM cells. Although no direct experimental evidence is available as yet, our results are consistent with the hypothesis that the functioning of a protein kinase activity may be necessary for transcriptional induction of genes by double-stranded RNA and for gene induction by interferons in those cells in which signal 1 production is needed.
Full text
PDF


Images in this article
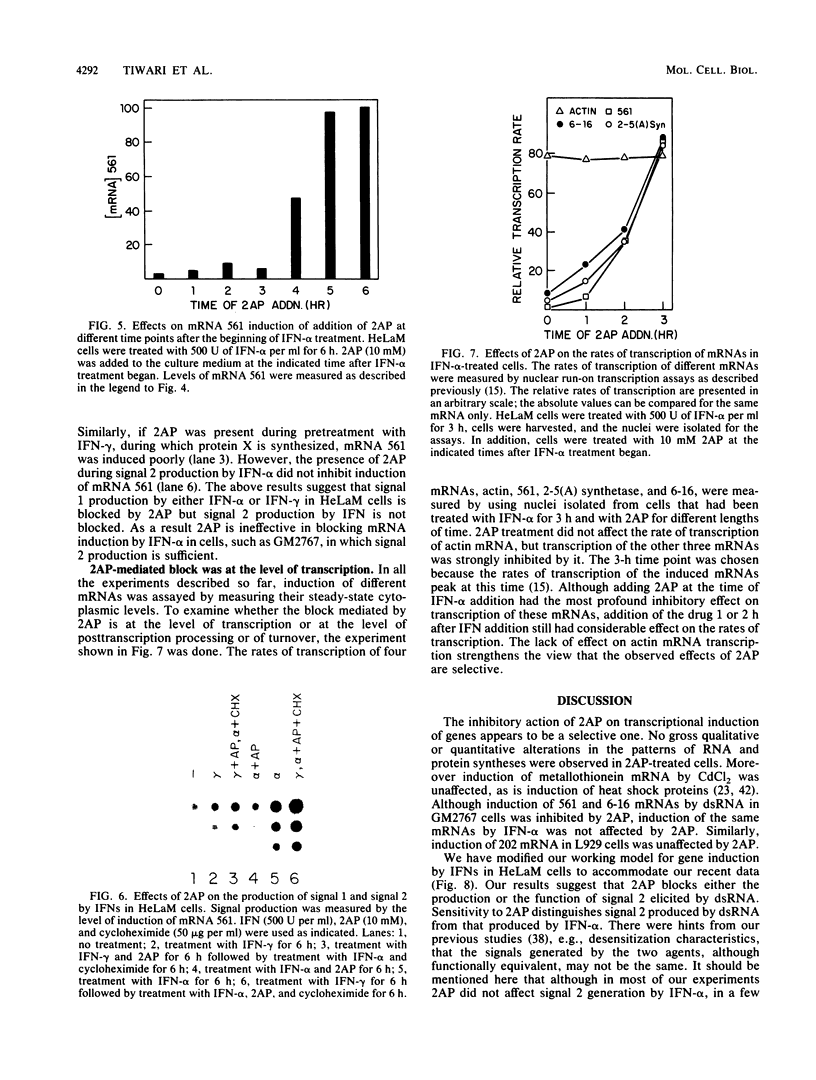
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
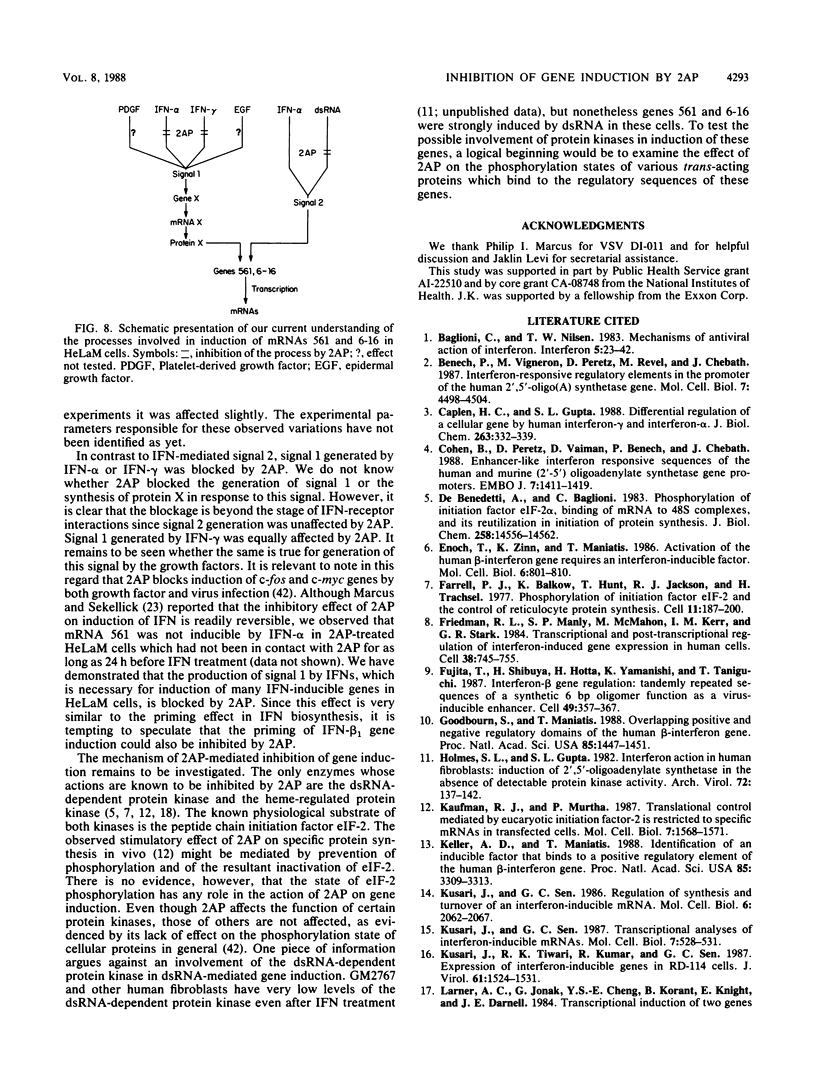
- Baglioni C., Nilsen T. W. Mechanisms of antiviral action of interferon. Interferon. 1983;5:23–42. [PubMed] [Google Scholar]
- Benech P., Vigneron M., Peretz D., Revel M., Chebath J. Interferon-responsive regulatory elements in the promoter of the human 2',5'-oligo(A) synthetase gene. Mol Cell Biol. 1987 Dec;7(12):4498–4504. doi: 10.1128/mcb.7.12.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourrie B. J., Casellas P., Blythman H. E., Jansen F. K. Study of the plasma clearance of antibody--ricin-A-chain immunotoxins. Evidence for specific recognition sites on the A chain that mediate rapid clearance of the immunotoxin. Eur J Biochem. 1986 Feb 17;155(1):1–10. doi: 10.1111/j.1432-1033.1986.tb09451.x. [DOI] [PubMed] [Google Scholar]
- Caplen H. S., Gupta S. L. Differential regulation of a cellular gene by human interferon-gamma and interferon-alpha. J Biol Chem. 1988 Jan 5;263(1):332–339. [PubMed] [Google Scholar]
- Cohen B., Peretz D., Vaiman D., Benech P., Chebath J. Enhancer-like interferon responsive sequences of the human and murine (2'-5') oligoadenylate synthetase gene promoters. EMBO J. 1988 May;7(5):1411–1419. doi: 10.1002/j.1460-2075.1988.tb02958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Benedetti A., Baglioni C. Phosphorylation of initiation factor eIF-2 alpha, binding of mRNA to 48 S complexes, and its reutilization in initiation of protein synthesis. J Biol Chem. 1983 Dec 10;258(23):14556–14562. [PubMed] [Google Scholar]
- Enoch T., Zinn K., Maniatis T. Activation of the human beta-interferon gene requires an interferon-inducible factor. Mol Cell Biol. 1986 Mar;6(3):801–810. doi: 10.1128/mcb.6.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell P. J., Balkow K., Hunt T., Jackson R. J., Trachsel H. Phosphorylation of initiation factor elF-2 and the control of reticulocyte protein synthesis. Cell. 1977 May;11(1):187–200. doi: 10.1016/0092-8674(77)90330-0. [DOI] [PubMed] [Google Scholar]
- Friedman R. L., Manly S. P., McMahon M., Kerr I. M., Stark G. R. Transcriptional and posttranscriptional regulation of interferon-induced gene expression in human cells. Cell. 1984 Oct;38(3):745–755. doi: 10.1016/0092-8674(84)90270-8. [DOI] [PubMed] [Google Scholar]
- Fujita T., Shibuya H., Hotta H., Yamanishi K., Taniguchi T. Interferon-beta gene regulation: tandemly repeated sequences of a synthetic 6 bp oligomer function as a virus-inducible enhancer. Cell. 1987 May 8;49(3):357–367. doi: 10.1016/0092-8674(87)90288-1. [DOI] [PubMed] [Google Scholar]
- Goodbourn S., Maniatis T. Overlapping positive and negative regulatory domains of the human beta-interferon gene. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1447–1451. doi: 10.1073/pnas.85.5.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes S. L., Gupta S. L. Interferon action in human fibroblasts: induction of 2', 5'-oligoadenylate synthetase in the absence of detectable protein kinase activity. Arch Virol. 1982;72(1-2):137–142. doi: 10.1007/BF01314459. [DOI] [PubMed] [Google Scholar]
- Kaufman R. J., Murtha P. Translational control mediated by eucaryotic initiation factor-2 is restricted to specific mRNAs in transfected cells. Mol Cell Biol. 1987 Apr;7(4):1568–1571. doi: 10.1128/mcb.7.4.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller A. D., Maniatis T. Identification of an inducible factor that binds to a positive regulatory element of the human beta-interferon gene. Proc Natl Acad Sci U S A. 1988 May;85(10):3309–3313. doi: 10.1073/pnas.85.10.3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusari J., Sen G. C. Regulation of synthesis and turnover of an interferon-inducible mRNA. Mol Cell Biol. 1986 Jun;6(6):2062–2067. doi: 10.1128/mcb.6.6.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusari J., Sen G. C. Transcriptional analyses of interferon-inducible mRNAs. Mol Cell Biol. 1987 Jan;7(1):528–531. doi: 10.1128/mcb.7.1.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusari J., Tiwari R. K., Kumar R., Sen G. C. Expression of interferon-inducible genes in RD-114 cells. J Virol. 1987 May;61(5):1524–1531. doi: 10.1128/jvi.61.5.1524-1531.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legon S., Brayley A., Hunt T., Jackson R. J. The effect of cyclic AMP and related compounds on the control of protein synthesis in reticulocyte lysates. Biochem Biophys Res Commun. 1974 Feb 4;56(3):745–752. doi: 10.1016/0006-291x(74)90668-8. [DOI] [PubMed] [Google Scholar]
- Lengyel P. Biochemistry of interferons and their actions. Annu Rev Biochem. 1982;51:251–282. doi: 10.1146/annurev.bi.51.070182.001343. [DOI] [PubMed] [Google Scholar]
- Levy D. E., Kessler D. S., Pine R., Reich N., Darnell J. E., Jr Interferon-induced nuclear factors that bind a shared promoter element correlate with positive and negative transcriptional control. Genes Dev. 1988 Apr;2(4):383–393. doi: 10.1101/gad.2.4.383. [DOI] [PubMed] [Google Scholar]
- Luster A. D., Unkeless J. C., Ravetch J. V. Gamma-interferon transcriptionally regulates an early-response gene containing homology to platelet proteins. Nature. 1985 Jun 20;315(6021):672–676. doi: 10.1038/315672a0. [DOI] [PubMed] [Google Scholar]
- Marcus P. I., Sekellick M. J. Defective interfering particles with covalently linked [+/-]RNA induce interferon. Nature. 1977 Apr 28;266(5605):815–819. doi: 10.1038/266815a0. [DOI] [PubMed] [Google Scholar]
- Marcus P. I., Sekellick M. J. Interferon induction by viruses. XVI. 2-Aminopurine blocks selectively and reversibly an early stage in interferon induction. J Gen Virol. 1988 Jul;69(Pt 7):1637–1645. doi: 10.1099/0022-1317-69-7-1637. [DOI] [PubMed] [Google Scholar]
- Merlin G., Chebath J., Benech P., Metz R., Revel M. Molecular cloning and sequence of partial cDNA for interferon-induced (2'-5')oligo(A) synthetase mRNA from human cells. Proc Natl Acad Sci U S A. 1983 Aug;80(16):4904–4908. doi: 10.1073/pnas.80.16.4904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestka S., Langer J. A., Zoon K. C., Samuel C. E. Interferons and their actions. Annu Rev Biochem. 1987;56:727–777. doi: 10.1146/annurev.bi.56.070187.003455. [DOI] [PubMed] [Google Scholar]
- Porter A. C., Chernajovsky Y., Dale T. C., Gilbert C. S., Stark G. R., Kerr I. M. Interferon response element of the human gene 6-16. EMBO J. 1988 Jan;7(1):85–92. doi: 10.1002/j.1460-2075.1988.tb02786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragg H., Weissmann C. Not more than 117 base pairs of 5'-flanking sequence are required for inducible expression of a human IFN-alpha gene. Nature. 1983 Jun 2;303(5916):439–442. doi: 10.1038/303439a0. [DOI] [PubMed] [Google Scholar]
- Reich N., Evans B., Levy D., Fahey D., Knight E., Jr, Darnell J. E., Jr Interferon-induced transcription of a gene encoding a 15-kDa protein depends on an upstream enhancer element. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6394–6398. doi: 10.1073/pnas.84.18.6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin B. Y., Anderson S. L., Lunn R. M., Hellermann G. R., Richardson N. K., Smith L. J. Production of a monoclonal antibody directed against an interferon-induced 56,000-dalton protein and its use in the study of this protein. J Virol. 1988 Jun;62(6):1875–1880. doi: 10.1128/jvi.62.6.1875-1880.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford M. N., Hannigan G. E., Williams B. R. Interferon-induced binding of nuclear factors to promoter elements of the 2-5A synthetase gene. EMBO J. 1988 Mar;7(3):751–759. doi: 10.1002/j.1460-2075.1988.tb02872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanta H., Engel D. A., Chao H. M., Thakur A., García-Blanco M. A., Lengyel P. Interferons as gene activators. Cloning of the 5' terminus and the control segment of an interferon activated gene. J Biol Chem. 1986 Sep 5;261(25):11849–11858. [PubMed] [Google Scholar]
- Saunders M. E., Gewert D. R., Tugwell M. E., McMahon M., Williams B. R. Human 2-5A synthetase: characterization of a novel cDNA and corresponding gene structure. EMBO J. 1985 Jul;4(7):1761–1768. doi: 10.1002/j.1460-2075.1985.tb03848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen G. C. Biochemical pathways in interferon-action. Pharmacol Ther. 1984;24(2):235–257. doi: 10.1016/0163-7258(84)90036-6. [DOI] [PubMed] [Google Scholar]
- Staeheli P., Danielson P., Haller O., Sutcliffe J. G. Transcriptional activation of the mouse Mx gene by type I interferon. Mol Cell Biol. 1986 Dec;6(12):4770–4774. doi: 10.1128/mcb.6.12.4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari R. K., Kusari J., Sen G. C. Functional equivalents of interferon-mediated signals needed for induction of an mRNA can be generated by double-stranded RNA and growth factors. EMBO J. 1987 Nov;6(11):3373–3378. doi: 10.1002/j.1460-2075.1987.tb02659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wathelet M. G., Clauss I. M., Nols C. B., Content J., Huez G. A. New inducers revealed by the promoter sequence analysis of two interferon-activated human genes. Eur J Biochem. 1987 Dec 1;169(2):313–321. doi: 10.1111/j.1432-1033.1987.tb13614.x. [DOI] [PubMed] [Google Scholar]
- Weissmann C., Weber H. The interferon genes. Prog Nucleic Acid Res Mol Biol. 1986;33:251–300. doi: 10.1016/s0079-6603(08)60026-4. [DOI] [PubMed] [Google Scholar]
- Zinn K., Keller A., Whittemore L. A., Maniatis T. 2-Aminopurine selectively inhibits the induction of beta-interferon, c-fos, and c-myc gene expression. Science. 1988 Apr 8;240(4849):210–213. doi: 10.1126/science.3281258. [DOI] [PubMed] [Google Scholar]
- Zullo J. N., Cochran B. H., Huang A. S., Stiles C. D. Platelet-derived growth factor and double-stranded ribonucleic acids stimulate expression of the same genes in 3T3 cells. Cell. 1985 Dec;43(3 Pt 2):793–800. doi: 10.1016/0092-8674(85)90252-1. [DOI] [PubMed] [Google Scholar]