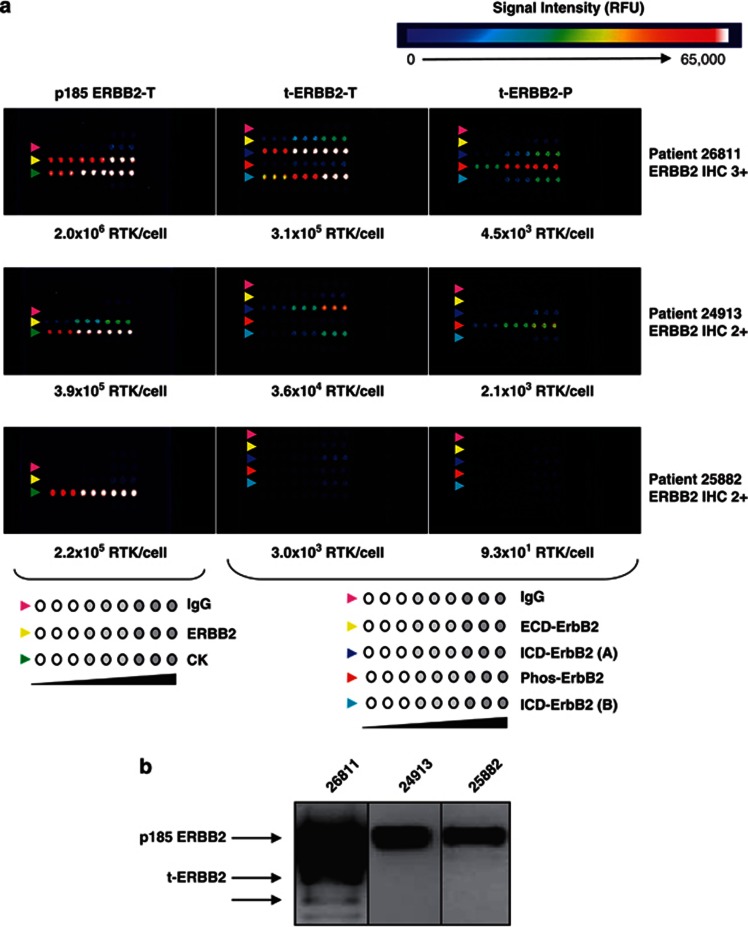
Figure 2.
Detection of ERBB2 isoforms in primary human breast tumor samples. (a) CEER analysis of human tumor samples reveals the presence and phosphorylation of t-ERBB2s. Lysates of tumors from three patients with high (no. 26811) or intermediate (nos. 24913 and 25882) ERBB2 expression as scored by IHC were tested. Assay configuration is illustrated below the CEER panels. Antibodies were arrayed in triplicate at three concentrations. Left panel antibodies for total p185 ERBB2 assay: control IgG (pink arrows), ECD-ERBB2 (yellow arrows) and cytokeratin (CK, green arrows). Center and right panel antibodies for total and phosphorylated t-ERBB2 assay: control IgG (pink arrows), ECD-ERBB2 (yellow arrows), ICD-ERBB2 (dark and light blue arrows) and phosphorylated ERBB2 (red arrows). Before t-ERBB2 CEER assay (center and right panels), full-length p185 ERBB2 was removed with an N-terminal ECD-directed antibody. (b) Western blot analysis of ERBB2 isoforms in human breast tumor samples with varying levels of ERBB2 (by IHC) assayed in part (a). Patient specimen lysates 26811, 24913 and 25882 were probed with antibody against ICD-ERBB2. Patient 26811 with positive western analysis also showed high level of ERBB2 as well as t-ERBB2 expression by CEER, with the use of much less cell lysate. Using CEER configuration, a quantitative detection of full-length ERBB2 and t-ERBB2 was achieved in samples (24913 and 25882) with IHC2+ status. Of note, t-ERBB2 was not detected by western blot analysis in these samples. Furthermore, a robust phosphorylation of t-ERBB2 was observed in patient 26811, whereas lower level was detected in patient 24913.