Figure 4.
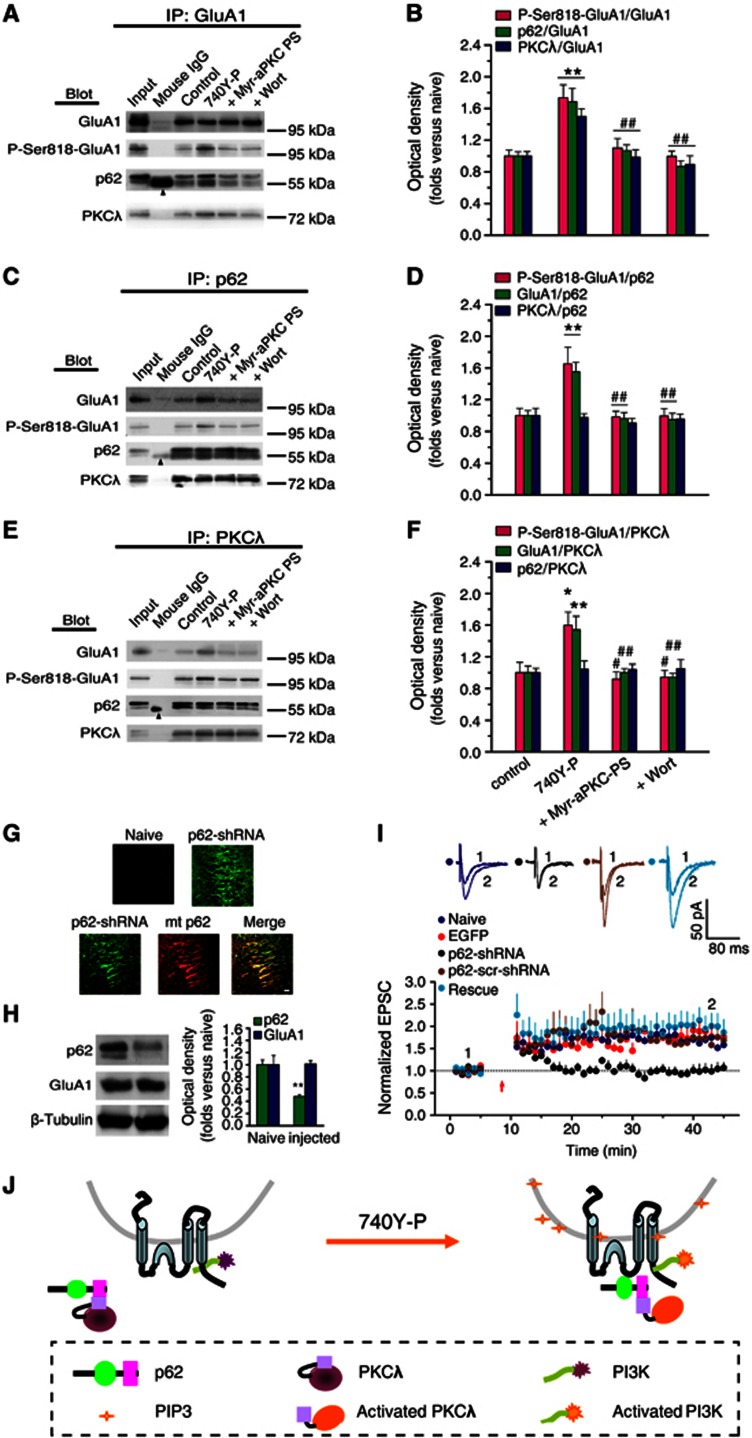
p62 serves as a scaffold protein to associate with both PKCλ and AMPAR during PI3K-induced LTP. (A, C, E) Co-IP assay with anti-GluA1, p62 or PKCλ antibody revealed the association of GluA1 with p62 or with PKCλ under various treatments. Arrowhead indicates the non-immune IgG heavy chain. (B, D, F) Statistical plot of data displaying the effects of various treatments on the association of GluA1 with p62 or with PKCλ as shown in (A), (C) and (E), respectively. PI3K activation potentiated the association between p62 and GluA1 or the association between PKCλ and GluA1 (p62/GluA1, 1.69±0.17, n=9, compared with control, P<0.01; PKCλ/GluA1, 1.50±0.09, n=9, compared with control, P<0.01; one-way ANOVA LSD test; A and B, also seen in C–F), but not affected the association between PKCλ and p62 (0.98±0.05, n=9; compared with control, P>0.05; C and D, also seen in E and F). The amount of P-Ser818-GluA1 was also increased simultaneously (P818-GluA1/GluA1, 1.74±0.16, n=5, compared with control, P<0.01; A and B, also seen in C–F). The potentiation was antagonized by blocking PKCλ with Myr-aPKC-PS (p62/GluA1, 1.07±0.07, n=9, compared with 740Y-P, P<0.01; PKCλ/GluA1, 0.99±0.09, n=9, compared with 740Y-P, P<0.01; P818-GluA1/GluA1, 1.10±0.11, n=5, compared with 740Y-P, P<0.01; A and B, also seen in C–F) or by PI3K antagonist Wort (p62/GluA1, 0.86±0.07, n=9, compared with 740Y-P, P<0.01; PKCλ/GluA1, 0.90±0.11, n=9, compared with 740Y-P, P<0.01; P818-GluA1/GluA1, 1.00±0.07, n=5, compared with 740Y-P, P<0.01; A and B, also seen in C–F. *Compared with control, P<0.05; **compared with control, P<0.01; #compared with 740Y-P, P<0.05; ##compared with 740Y-P, P<0.01. (G) Images showing EGFP expression in dorsal hippocampal slices prepared from p62-shRNA injected and contralateral (naive) hemispheres following unilateral injections (4 μl) of an rAAV coexpressing p62-shRNA and EGFP. A rescue experiment was also performed in parallel on slices prepared from hemisphere injected with both EGFP-p62-shRNA (green) and mcherry-p62 (red; bottom). Scale bar: 20 μm. (H) Confirmation of PKCλ knockdown. A 52% (52%±3%) reduction in p62 was observed in preparations from dorsal hippocampus. (I) p62 knockdown led to impaired LTP expression (1.03±0.13, n=9; compared with baseline, P>0.05, paired sample t test; compared with uninjected side cells, 1.63±0.05, n=6, P<0.01, one-way ANOVA LSD test), which was reversed by PKCλ rescue (1.99±0.29, n=5; compared with p62 KD, P<0.01; compared with uninjected or EGFP control, P>0.05). As a control, LTP was normal in cells transfected with p62-scr-shRNA (1.65±0.12, n=4; compared with baseline, P<0.05; compared with p62 KD, P<0.05). The EGFP control was borrowed from Figure 1C for comparison. (J) A schematic illustration describes the possible ‘briding’ role of p62 during PI3K-induced LTP. Under basal conditions, PKCλ/p62 association is already at high level, while relatively fewer GluA1 is associated with p62/PKCλ complex. PI3K activation by 740Y-P treatment causes accumulation of its products, PIP3, on the membrane of AMPAR-containing vesicles. PIP3 can thus activate downstream PKCλ, which in turn trigger the recruitment of the p62/PKCλ complex to vicinity of GluA1 via an unknown mechanism, thereby facilitating direct interaction between GluA1 and PKCλ. Thus, p62 may function as a ‘bridge’ between AMPAR and PKCλ to facilitate direct GluA1 phosphorylation by PKCλ.
Source data for this figure is available on the online supplementary information page.