Figure 5.
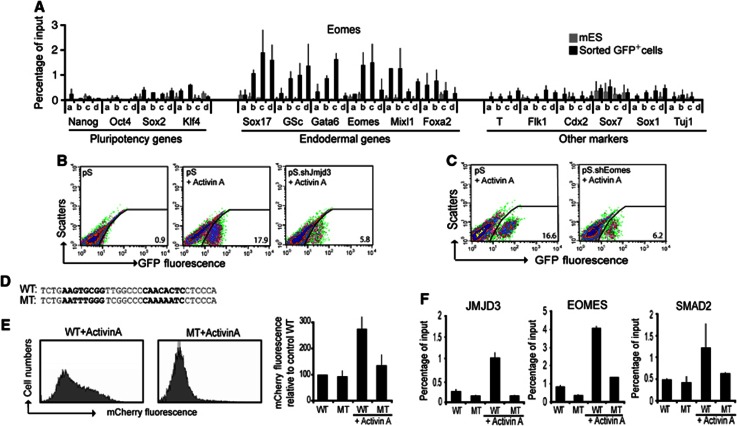
Eomes recruits Jmjd3 and Smad2 to the promoter regions of core definitive endoderm genes. (A) Eomes selectively occupies the promoters of definitive endoderm genes in sorted Sox17.GFP+ cells. ChIP experiments were using four pairs of primers at the proximal-promoter regions (a–d) of select promoters from three groups; core pluripotency regulators, core definitive endoderm, and key transcriptional regulators of other germ layers. (B, C) Definitive endoderm specification by Activin A requires both Eomes and Jmjd3. (B) The number of GFP+ cells formed was analysed by flow cytometry in control (pS) cells differentiated without Activin A (left panel), with Activin A (middle panel), and in Jmjd3-knockdown (pS.shJmjd3) cells differentiated with Activin A (right panel). (C) Flow cytometry was performed in control (pS) cells (left panel) and Eomes-knockdown cells (pS.shEomes), both differentiated with Activin A (right panel). (D–F) Eomes-Smad2-Jmjd3 binding requires the T-box binding motif on the promoter of Sox17. (D) Sequences of the wild-type and the mutated Eomes binding motif on Sox17 promoter, containing two tandem core motifs (in bold). (E) mCherry fluorescence in Hek293T transfected with the Sox17 promoter mcherry reporter constructs containing the wild-type Eomes binding sequence motif (left panel) was higher than the mutant one (middle panel) following stimulation with Activin A. The graph shows mean±s.e.m. of the relative mcherry fluorescence from two independent experiments. (F) ChIP analyses of JMJD3, EOMES, and SMAD2 show increased binding to the wild-type but not to the mutated Sox17 promoter constructs in Hek293T following Activin A treatment. Data values are mean±s.e.m. (n=2–3).