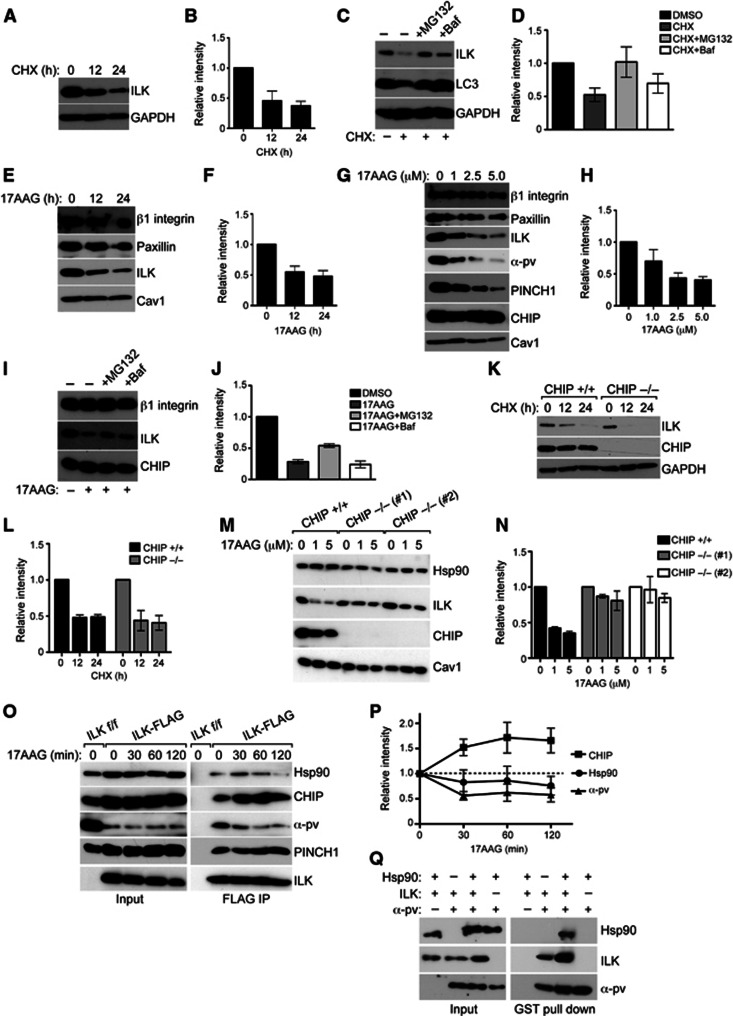
Figure 5.
Hsp90 stabilizes ILK and protects it from CHIP-mediated degradation. (A) Western blot of fibroblasts treated with CHX to stop protein synthesis for time points indicated. Majority of ILK is degraded in 24 h. GAPDH is used as loading control. (B) Quantification of ILK levels from experiments from panel A. Data are presented as mean±s.e.m., n=3. (C) Western blot of fibroblasts treated with CHX to stop protein synthesis for time points indicated. Inhibition of proteasome with MG132 or inhibition of lysosome with Baf retards ILK degradation. LC3 is a positive control for inhibition of lysosome; GAPDH is used as loading control. (D) Quantification of ILK levels from experiments from C. Data are presented as mean±s.e.m., n=3. (E) Western blot of fibroblasts treated with 5 μM 17AAG to inhibit Hsp90 for time points indicated. Inhibition of Hsp90 leads to downregulation of ILK levels. Other focal adhesion proteins such as paxillin or β1 integrin are unaffected. Caveolin1 (Cav1) is used as a loading control. (F) Quantification of ILK levels from experiments from E. Data are presented as mean±s.e.m., n=3. (G) Western blot of fibroblasts treated with increasing concentrations of 17AAG. Inhibition of Hsp90 leads to dose-dependent downregulation of ILK levels together with PINCH1 and α-parvin. Paxillin or β1 integrin are unaffected. (H) Quantification of ILK levels from experiments from G. Data are presented as mean±s.e.m., n=3. (I) Western blot of fibroblasts treated with 17AAG in the presence of MG132 or Baf. Blocking the proteasome with MG132 inhibits 17AAG-induced degradation of ILK. (J) Quantification of ILK levels from experiments from I. Data are presented as mean±s.e.m., n=3. (K) Western blot of CHIP+/+ and CHIP−/− fibroblasts treated with CHX for time points indicated. ILK levels are reduced to a comparable extent in CHIP+/+ and CHIP−/− fibroblasts. (L) Quantification of ILK levels from experiments from K. Data are presented as mean±s.e.m., n=3. (M) Western blot of CHIP+/+ and CHIP−/− fibroblasts from two independent isolations treated with 17AAG. Inhibition of Hsp90 leads to downregulation of ILK levels. This effect is attenuated in CHIP−/− cells. (N) Quantification of ILK levels from experiments from M. Data are presented as mean±s.e.m., n=3. (O) Western blot of FLAG immunoprecipitates from ILK-FLAG expressing cells treated with 17AAG. Inhibition of Hsp90 leads to an increase in the interaction of ILK with CHIP and a dissociation of ILK from Hsp90 and α-parvin. Interaction with PINCH1 is not affected. (P) Quantification of experiments from O. Data are presented as mean±s.e.m., n=3. (Q) Western blot of GST pull down with recombinant GST-tagged α-parvin together with recombinant ILK and Hsp90. ILK co-precipitates with α-parvin, whereas Hsp90 co-precipitates with α-parvin only in the presence of ILK. Presence of Hsp90 enhances the interaction between ILK and α-parvin.
Source data for this figure is available on the online supplementary information page.