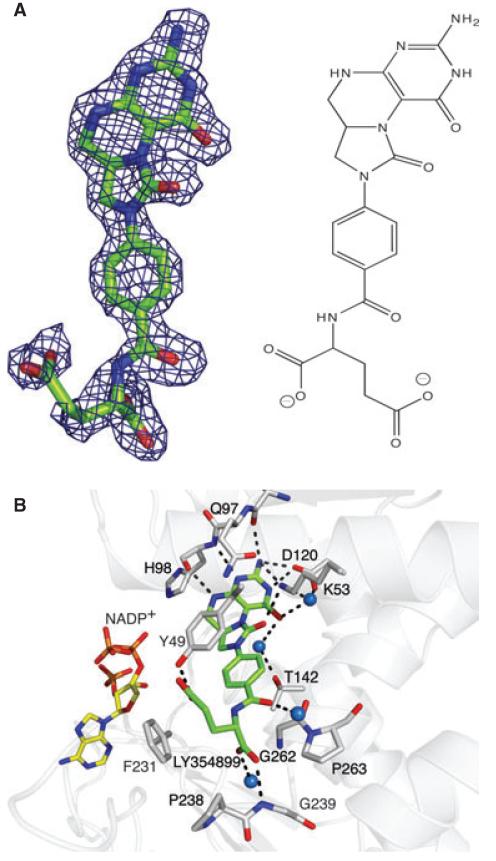
Fig. 3.
LY354899 bound to AbFolD. (A) The Fo − Fc omit map (blue chicken wire) contoured at 2σ and the chemical structure of LY354899. (B) Details of binding in the active site. Residues and waters (blue spheres) that interact are labelled. Dashed bonds represent potential hydrogen-bonding associations. The fragment of the cofactor that could be modelled is shown as sticks with atomic positions coloured yellow for C, blue for N, orange for P and red for O. The C atoms of LY354899 and the FolD residues are green and grey, respectively.