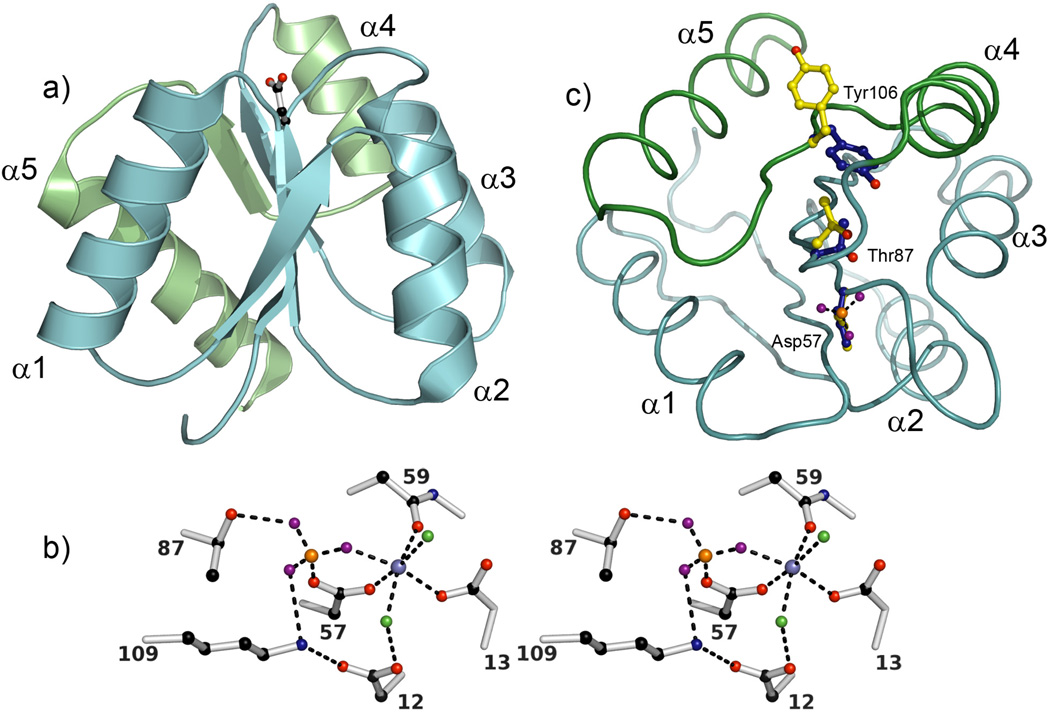
Figure 2.
Conserved features of RR receiver domains. (a) A ribbon diagram of E. coli CheY (1FQW) displays the classic (βα)5 fold of receiver domains with the site of phosphorylation (Asp57) shown in ball-and-stick mode. The α4-β5-α5 signaling face, the region that shows the largest structural perturbations upon phosphorylation, is colored green. (b) A stereo diagram of the active site of CheY (1FWQ) illustrates the roles of highly conserved residues in coordinating the phosphate and divalent metal ion. Due to the lability of the acyl phosphate, structural analyses of active RRs have often been carried out in the presence of the non-covalent phosphoryl analog beryllofluoride [65]. The BeF3− complex (beryllium, orange; fluorides, purple), which coordinates to Asp57, is stabilized by interactions with the side chains of Lys109 and Thr87, and a divalent metal ion (metallic blue). The metal ion (Mn2+ in this structure) is required for catalysis of both phosphotransfer and autodephosphorylation and is positioned by octahedral coordination to the side chains of Asp12 (water-mediated), Asp13, and Asp57, the backbone carbonyl of Asn59, a fluoride of BeF3−, and an additional water molecule (green). (c) RRs utilize a common mechanism to couple phosphorylation to surface changes. This mechanism involves the reorientation of two highly conserved residues, a hydroxyl-containing residue (Ser/Thr) and an aromatic residue (Phe/Tyr). The relative orientations of these side chains (Thr87 and Tyr106 in CheY) and that of the phosphorylated aspartate (Asp57) in their inactive (yellow) and active (blue) conformations are shown in ball-and-stick mode following superpositioning of inactive and active CheY structures (2CHE and 1FWQ). In the inactive conformation, the Ser/Thr and Phe/Tyr are oriented away from the active site with the aromatic side chain in a surface exposed position on the α4-β5-α5 face. In the active conformation, both side chains are oriented towards the active site with the Phe/Tyr side chain buried and the hydroxyl group of the Ser/Thr forming a hydrogen bond with a phosphate oxygen (in this structure, a fluoride of BeF3−). The view represents a rotation of ~90° about the x-axis relative to the view in (a) and a Cα trace of active CheY with colors as in (a) is shown for reference. In all panels, oxygen atoms are colored red; nitrogen atoms, blue; and carbon atoms, black, unless noted otherwise.