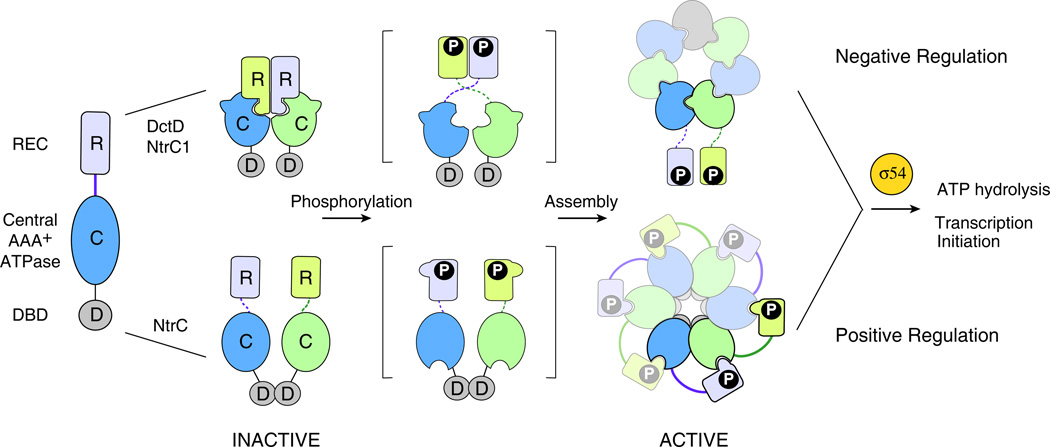
Figure 3.
Activation mechanisms of NtrC subfamily RRs. NtrC subfamily members share a similar domain composition of receiver (R), ATPase (C) and DNA-binding (D) domains and are all dependent on the ring assembly of the central ATPase domains (alternating blue and green) to interact with σ54 for transcription regulation. However, despite these similarities, two distinct assembly mechanisms have been discovered within the subfamily so far. In DctD and NtrC1 (top), the central ATPase domain is intrinsically competent for ring assembly but the inactive receiver domain negatively regulates assembly by holding the ATPase (C) domains in a front-to-front dimer, unfavorable for a front-to-back assembly [50]. Phosphorylation or removal of the receiver domain exposes the surface that is buried in the inactive dimer, relieving the inhibition. In NtrC (bottom), the isolated ATPase domain lacks significant ATPase activity. Phosphorylation causes conformational changes that enable the receiver domain from one subunit to be in contact with the ATPase domain of a second subunit, stabilizing the ring structure and promoting ATPase activity [49].