Figure 2. N-Glycosylation status of AMPA receptor subunits in DLPFC in schizophrenia.
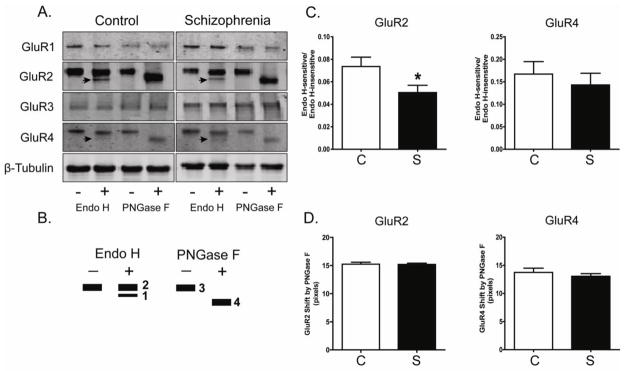
A. Representative immunoblots from DLPFC showing changes in electrophoretic mobility for GluR2 and GluR4, but not GluR1, GluR3 or β-tubulin following deglycosylation with Endo H or PNGase F. B. A schematic diagram of band patterns following deglycosylation with either Endo H or PNGase F. Endo H removes only immature, high mannose-containing or hybrid sugars, while PNGase F removes all N-linked sugars, thus leading to full N-linked deglycosylation. C. Quantification of Endo H-sensitive molecular mass shifts for GluR2 and GluR4. Graphs show the ratio of measured intensities of the Endo H-sensitive band to the Endo H-insensitive band (Bands 1 and 2, respectively, in Panel B). GluR2 was significantly less sensitive to Endo H in schizophrenia (S; N=35) versus the comparison group (C; N=31). *P<0.05. D. Quantification of PNGase F-sensitive molecular mass shifts for GluR2 and GluR4. Data are presented as the distance between the PNGase F deglycosylated protein band of higher mobility (Band 4 in Panel B), and the same protein without PNGase F treatment (Band 3 in Panel B). The molecular mass shift caused by PNGase F deglycosylation was not different between schizophrenia and comparison subjects.
