Abstract
Developmental dyslexia is a neurobiological deficit characterized by persistent difficulty in learning to read in children and adults who otherwise possess normal intelligence. Functional and structural connectivity data suggest that developmental dyslexia could be a disconnection syndrome. However, whether abnormalities in connectivity exist in beginning readers at-risk for reading difficulties is unknown. Using graphtheoretical analysis, we investigated differences in global and regional topological properties of structural brain networks in 42 beginning readers with (FH+) and without (FH−) familial risk for reading difficulties. We constructed separate structural correlation networks based on measures of surface area and cortical thickness. Results revealed changes in topological properties in brain regions known to be abnormal in dyslexia (left supramarginal gyrus, left inferior frontal gyrus) in the FH+ group mainly in the network constructed from measures of cortical surface area. We also found alterations in topological properties in regions that are not often advertised as dyslexia but nonetheless play important role in reading (left posterior cingulate, hippocampus, and left precentral gyrus). To our knowledge, this is the first report of altered topological properties of structural correlation networks in children at risk for reading difficulty, and motivates future studies that examine the mechanisms underlying how these brain networks may mediate the influences of family history on reading outcome.
Keywords: Reading difficulty, Dyslexia, Learning disability, Graph analysis, Small-world, Brain networks
Introduction
Developmental dyslexia, the most common form of all learning disabilities, is a neurobiological deficit characterized by persistent difficulty in learning to read (Shaywitz, 1998; Shaywitz et al., 2003). While the neurobiological etiology of developmental dyslexia is a matter of debate, the most accepted model, derived from both functional and structural neuroimaging studies reporting impairment in the left temporo-parietal (including the inferior parietal lobule) and occipito-temporal regions, suggests a dysfunction in neural circuits associated with phonological and orthographic processing (Eckert et al., 2005; Hoeft et al., 2006, 2007; van der Mark et al., 2009) [see (Maisog et al., 2008; Richlan et al., 2009) for a review]. These regions are also abnormal in beginning readers at familial risk for reading difficulties (Brem et al., 2010; Maurer et al., 2007; Raschle et al., 2011, 2012; Specht et al., 2009).
Recent data suggest that developmental dyslexia could be a disconnection syndrome. Specifically, individuals with developmental dyslexia have shown disrupted functional connectivity in the language-dominant left-hemisphere (Horwitz et al., 1998; Pugh et al., 2000; van der Mark et al., 2011), altered effective connectivity between the left-hemisphere language regions and bilateral frontal regions (Cao et al., 2008), and decreased fractional anisotropy in the left-hemisphere white matter tracts, fronto-temporal and temporo-parietal white matter (Frye et al., 2010a, 2010c; Odegard et al., 2009; Steinbrink et al., 2008). This body of evidence suggests that developmental dyslexia is associated with alterations in connectivity of diffuse regions that might affect the topological properties of brain networks.
An abundance of research has shown that dyslexia is highly familial [see (Petryshen and Pauls, 2009) for a review]. Children with family history of reading difficulties have a 34–65% chance of developing dyslexia (Pennington and Lefly, 2001). Therefore, alterations in brain networks might be evident in beginning readers at familial risk for reading difficulties. In the present study, we applied graph theoretical analysis to investigate differences in global and regional topological properties of structural brain networks in children with and without familial risk for reading difficulties as they were beginning formal reading instruction in kindergarten. The unique feature of graphtheoretical analysis, compared with traditional connectivity analysis, is that it provides a unique framework to directly test the differences in topological properties of brain networks. Coordinated variations in brain morphology have been proposed as a valid measure to infer large-scale structural brain networks (He et al., 2007). The structural networks constructed from morphometric correlations of cortical volume, thickness, and surface data are consistent with those constructed from tract-tracing data (Bernhardt et al., 2008; He et al., 2007; Lerch et al., 2006; Sanabria-Diaz et al., 2010), and have been shown to follow small-world characteristics in healthy individuals (Bassett et al., 2008; Chen et al., 2008; Fan et al., 2011; He and Evans, 2010). A small-world architecture reflects a network that is simultaneously highly segregated and integrated and allows more efficient rates of information processing and learning (Simard and Nadeau, 2005). Since quantitative description of small-worldness has been presented for brain networks (Watts and Strogatz, 1998), a large number of studies have shown alterations in topological properties of brain structural networks associated with Alzheimer's disease, schizophrenia, and multiple sclerosis (Bassett et al., 2008; He et al., 2008, 2009).
While still under debate, recent evidence suggests that the two independent measures of cortical volume, i.e. cortical surface area and thickness, may be driven by distinct cellular mechanisms that are genetic in their origins (Eyler et al., 2011; Kapellou et al., 2006; Rimol et al., 2010; Sanabria-Diaz et al., 2010). Perhaps driven by these effects, previous neuroimaging studies found more pronounced association between alterations in surface-area measures, rather than thickness measures, and history of dyslexia (Frye et al., 2010b) as well as severity of family history of reading difficulties (Black et al., 2012). Thus, we constructed separate structural networks based on surface area and cortical thickness data to further account for these mechanistic/genetic differences. We predicted alterations in topological properties in the left-hemisphere reading network that would be more pronounced in the surface area network.
Materials and Methods
Participants
A total of 42 (22 males, 38 right-handed) healthy, native Englishspeaking children (aged 5.59±0.39) participated in this study. Children (with and without family history of reading difficulties based originally on parental self-report) were recruited from local newspapers, school mailings (including both schools for children with learning disabilities and conventional schools), flyers, and mother's clubs. The participants' data used in this study is a subset of data used in our recent study (Black et al., 2012).
Family history of reading difficulty was operationally defined as parental report of reading disability in a first-degree relative (either biological parent or sibling). The children did not have any neurological or psychiatric disorders, were not on medication, and had no contraindications to MRI. The Stanford University Panel on Human Subjects in Medical Research approved the study and written informed consent was obtained from the parent/legal guardian of participants. Children were between 5 and 6 years of age and hence did not complete an assent form.
Assessment of family history and behavioral, cognitive and environmental measures
Once recruited, as in previous literature, family risk was assessed using the Adult Reading History Questionnaire (ARHQ) (Lefly and Pennington, 2000). An ARHQ cut-off score of 0.39 was used to determine the existence of family history based on previous literature (Black et al., 2012; Maurer et al., 2003, 2007, 2009) and in our prior paper where we established reliability and performed receiver operating characteristic (ROC) analysis using Test of Word Reading Efficiency Phonemic Decoding Efficiency (TOWRE PDE), a timed measure of reading non-words (Black et al., 2012). There were no significant correlations identified between ARHQ scores (maternal and paternal) and demographic information such as socio-economic status (SES) (or any of the individual factors such as education and occupation) or percentage of time spent with child generally and related to educational activities specifically (all p's>0.05) [refer to (Black et al., 2012) for the details].
SES was measured based on the procedure and questionnaire of Noble et al. (2006). Parents completed a brief questionnaire with items related to parental education, occupation and income. Parental education was defined as the average education of any parents (and step-parents/guardians) in the home. We used the 9-point Hollingshead Index Occupational Status Scale (Bornstein and Bradley, 2003) to score parental occupation, and used only the highest score of any parent, stepparent or guardian in the home. The income score was defined as the total family income divided by the official federal poverty threshold for a family of that size. Thus, for each family, an income-to-needs ratio was computed whereby the total family income was divided by official poverty threshold. Finally, a composite SES score was calculated for each child by factor analyzing the three scores (parental education, occupation, and income) using principal component analysis. A single principal component emerged, accounting for 57.1% of the variance. SES in our study refers to the factor loading score (mean 0; SD 1) that was computed for each child and entered into our subsequent analyses (Table 1).
Table 1.
Demographics of the two groups (FH+ vs. FH−).
| Measure | FH+ [mean (SD)] | FH− [mean (SD)] | T-Tests [t(1,40); p] | |
|---|---|---|---|---|
| Child | Age | 5.65 (0.46) | 5.51 (0.30) | 1.17; 0.25 |
| Gender | 9 female/13 male | 11 female/9 male | χ2 (1, N = 42) = 0.83, p=0.36 | |
| Socioeconomic status (SES) | 0.09 (0.94) | −0.15 (1.11) | 0.73; 0.468 | |
| Woodcock Johnson III brief intellectual ability-ss (IQ)a | 116.64 (10.86) | 121.95 (10.09) | 1.64; 0.109 | |
| Woodcock Johnson III visual matching-ss (Processing Speed-PS) | 104.05 (12.21) | 107.60 (11.07) | 0.99; 0.331 | |
| Woodcock reading mastery test letter ID-ss (LID) | 105.95 (8.73) | 115.50 (9.73) | 3.35; 0.002 | |
| Rapid automized naming color and object-ss (RAN) | 96.89 (17.07) | 101.30 (14.32) | 0.90; 0.372 | |
| CTOPP phonological awareness-cs (PA) | 106.45 (14.18) | 118.80 (12.15) | 3.01; 0.004 | |
| CTOPP phonological memory-cs (PM) | 104.27 (12.26) | 110.95 (11.02) | 1.85; 0.072 | |
| Total gray matter volume (TGMV) | 709.84 (65.53) | 711.88 (57.68) | 0.11; 0.916 | |
| Total white matter volume (TWMV) | 453.61 (46.29) | 451.31 (36.29) | 0.18; 0.859 | |
| HOMEb I (learning materials subscale) mean score | 0.87 (0.09) | 0.85 (0.08) | 0.73; 0.469 | |
| HOME II (language stimulation subscale) mean score | 0.98 (0.05) | 0.99 (0.44) | 0.42; 0.676 | |
| HOME V (academic stimulation subscale) mean score | 0.92 (0.28) | 1.00 (0.00) | 1.22; 0.230 | |
| FESc (achievement subscale) mean score | 2.99 (0.42) | 3.02 (0.52) | 0.19; 0.854 | |
| FES (intellectual-cultural subscale) mean score | 3.68 (0.57) | 3.77 (0.43) | 0.61; 0.547 | |
| Maternal | Age (Age-M) | 40.26 (4.12) | 37.58 (4.14) | 2.10; 0.042 |
| ARHQ (MFamRI) | 0.36 (0.17) | 0.25 (0.09) | 2.41; 0.021 | |
| Percent educational time with child (ET-M) | 68.86 (15.17) | 67.71 (21.15) | 0.20; 0.839 | |
| Education level (Ed-M) | 17.30 (1.91) | 17.13 (2.41) | 0.26; 0.800 | |
| WAIS-R-ss (IQ-M) | 112.00 (6.26) | 113.75 (4.82) | 1.01; 0.320 | |
| Paternal | Age (Age-P) | 42.83 (5.61) | 40.79 (5.07) | 1.21; 0.232 |
| ARHQ (PFamRI) | 0.40 (0.16) | 0.26 (0.09) | 3.55; 0.001 | |
| Percent educational time with child (ET-P) | 31.10 (15.16) | 32.27 (21.15) | 0.21; 0.836 | |
| Education level (Ed-P) | 16.36 (1.62) | 17.00 (2.64) | 0.95; 0.347 | |
| WAIS-R-ss (IQ-P) | 110.95 (6.55) | 113.37 (4.39) | 1.35; 0.186 | |
| Parental characteristics with history of reading disorder (RD) | ||||
| ARHQ (of parents with ARHQ≥0.4) | 0.52 (0.09) | N/A | ||
| Test of Word Reading Efficiency–Phonological Decoding-ss (TOWRE-PDE-ss) | 87.24 (10.99) | N/A | ||
| Parental characteristics without history of RD | ||||
| ARHQ (of parents with ARHQ≤0.4) | 0.25 (0.10) | 0.26 (0.6) | 0.26; 0.793 | |
| Test of Word Reading Efficiency–Phonological Decoding-ss (TOWRE-PDE-ss) | 95.53 (8.10) | 97.34 (8.40) | 0.63; 0.532 | |
ss represents standard score and cs represents composite score. Refer to Black et al., 2012 for further details of measurements.
Refers to the Home Observation for Measurement of the Environment.
Refers to the Family Environment Scale.
In addition, the Home Observation for Measurement of the Environment (HOME) Inventory (Caldwell and Bradley, 1984) was used to measure the quality and quantity of stimulation and support available to the child in the home environment. The focus of HOME is on the child in the environment, child as a recipient of inputs from objects, events, and transactions occurring in connection with the family surroundings. We did not find any significant difference between groups in learning materials, language stimulation and academic stimulation in the home (all p's>0.05) (Table 1).
Among those with a family history (FH+ group, N=22, age: mean, 5.65; SD, ±0.46, 3 left-handed), 13 children had paternal history, 9 had maternal history. In those without a family history (FH− group, N=20, age: mean, 5.51; SD, ±0.30, 1 left-handed), there was no reported history of reading impairment in any family member and both parents' ARHQ were less than 0.39. The demographics of the two family history groups (FH+ vs. FH−) (e.g., child age, parent age, SES, maternal and paternal education level, percentage of time spent with mother and father overall and related to education) were not significantly different (Table 1).
For parents without reading disabilities (as based on ARHQ less than 0.39) there were no significant differences between ARHQ scores and between the TOWRE PDE scores of the two groups (those labeled as FH+ and FH− but all without indication of reading disabilities as based on ARHQ less than 0.39) (Table 1).
It is noteworthy that the FH+ group showed significantly lower scores in phonological awareness, letter knowledge and verbal IQ compared with FH− group. These measures are known as predictors of reading outcome (Lyytinen et al., 2006, 2008; Puolakanaho et al., 2007), and hence it is no surprise that these measures were significantly lower in the FH+ group.
MRI data acquisition
Imaging data were acquired at the Richard M. Lucas Center for Imaging at Stanford University. Imaging data was acquired using GE Healthcare 3.0 Tesla 750 scanner 20.x software revision and an 8-channel phased array head coil (GE Healthcare, Waukesha, WI). Images acquired included an axial-oblique 3D T1-weighted sequence with the following parameters: fast spoiled gradient recalled echo (FSPGR) pulse sequence, inversion recovery preparation pulse TI= 400 ms; repetition time (TR)=8.5 ms; echo-time (TE)=3.4 ms; flip angle=15°; Receiver bandwidth +32 kHz; slice thickness= 1.2 mm; 128 slice locations; number of excitations (number of signals averaged)=1; field-of-view (FOV)=22 cm; Phase FOV=0.75; acquisition matrix=256×192; one scan per subject. The total time of scan was 4:34. To prepare for the scan, families received a packet of materials, including a CD of scanner noises and a DVD of a child going into a scanner, designed to desensitize him/her to the scanner sounds and environment. Children also participated in simulated MRI sessions at the center.
Image processing and cortical thickness and surface area extraction
Cortical reconstruction and volumetric segmentation were performed with the FreeSurfer image analysis suite version 5.0.0, which is documented and freely available for download online (http://surfer. nmr.mgh.harvard.edu/). The technical details of these procedures are described in prior publications (Dale and Fischl, 1999; Dale and Sereno, 1993; Fischl and Dale, 2000; Fischl et al., 1999a, 1999b, 2001, 2002, 2004a, 2004b; Han et al., 2006; Segonne et al., 2004). Briefly, this processing includes motion correction, removal of non-brain tissue using a hybrid watershed/surface deformation procedure (Segonne et al., 2004), automated Talairach transformation, segmentation of the subcortical white matter and deep gray matter volumetric structures (including hippocampus, amygdala, caudate, putamen, and ventricles) (Fischl et al., 2002, 2004a), intensity normalization (Sled et al., 1998), tessellation of the gray matter white matter boundary, automated topology correction (Fischl et al., 2001; Segonne et al., 2007), and surface deformation following intensity gradients to optimally place the gray/white (main) and gray/cerebrospinal fluid (pial) borders at the location where the greatest shift in intensity defines the transition to the other tissue class (Dale and Fischl, 1999; Dale and Sereno, 1993; Fischl and Dale, 2000). The main and pial surfaces were visually inspected, and where needed, the appropriate manual corrections were preformed as per the FreeSurfer Tutorial (http://surfer.nmr.mgh.harvard.edu/fswiki/FsTutorial). All FreeSurfer surfaces were inspected and adjusted by a single rater (blinded to subject group) with experience editing more than one hundred brains after passing an inter-rater reliability program with ≥0.95 (intraclass correlation coefficient) to gold-standard datasets for volumetric regions of interest. A senior staff member (MR) who was blind to the participant's FH status and who has inspected over one thousand FreeSurfer subjects verified all final surfaces.
Once the models are complete, a number of deformable procedures can be performed for further data processing and analysis including surface inflation (Fischl et al., 1999a), registration to a spherical atlas which utilized individual cortical folding patterns to match cortical geometry across subjects (Fischl et al., 1999b), parcellation of the cerebral cortex into units based on gyral and sulcal structure (Desikan et al., 2006; Fischl et al., 2004b), and creation of a variety of surface based data including maps of curvature and sulcal depth. This method uses both intensity and continuity information from the entire three dimensional MR volume in segmentation and deformation procedures to produce representations of cortical thickness, calculated as the closest distance from the gray/white boundary to the gray/CSF boundary at each vertex on the tessellated surface (Fischl and Dale, 2000). The maps are created using spatial intensity gradients across tissue classes and are therefore not simply reliant on absolute signal intensity. The maps produced are not restricted to the voxel resolution of the original data thus are capable of detecting sub-millimeter differences between groups. Procedures for the measurement of cortical thickness have been validated against histological analysis (Rosas et al., 2002) and manual measurements (Kuperberg et al., 2003; Salat et al., 2004). Freesurfer morphometric procedures show good test–retest reliability across scanner manufacturers and across field strengths (Han et al., 2006).
While previous studies adopting graph analysis used cortical thickness or cortical (subcortical) volumes as input, we also included cortical surface area and cerebellar volumes in the analyses, since thickness (surface area) allow us to examine distinct properties of interactions between brain regions as we describe below, and since cerebellar–cortical (subcortical) networks play important roles in reading development (Ben-Yehudah and Fiez, 2008). This analytic approach resulted in a total of 86 regions for each of the cortical thickness and surface area analyses (Desikan–Killiany Atlas) (Desikan et al., 2006). The names of the included regions are given in Fig. S1. Finally, surface areas, cortical thicknesses, and cerebellar and subcortical volumes were corrected for total surface area, mean cortical thickness, total cortical gray matter volume, and total subcortical gray matter volume, respectively, using linear regression analyses and obtaining residuals. Normalizations by correcting global measures in such a way remove the individual differences in morphometric measures affected by overall brain size and are a prerequisite for construction of structural correlation networks (Bernhardt et al., 2011; Fan et al., 2011; Hosseini et al., 2012b). These corrected measures were used for subsequent graph analyses.
Construction of structural correlation network
Data from 86 brain regions were used to construct surface area-based and cortical thickness-based structural correlation networks. For each group, an 86×86 correlation matrix R was generated with each entry rij defined as the Pearson correlation coefficient between the extracted residuals of regions i and j (Bernhardt et al., 2011; Fan et al., 2011; He et al., 2007). These morphometric correlations reflect connectivity, as axonally connected regions are believed to be influenced by common developmental, trophic and maturational effects (Bernhardt et al., 2011; Cheverud, 1984; Wright et al., 1999; Zhang and Sejnowsky, 2000). Previous studies have shown that the structural correlation networks are estimable with tens of subjects (Fan et al., 2011; Hosseini et al., 2012a, 2012b; van den Heuvel et al., 2009).
A binary adjacency matrix A was derived from each association matrix where aij was considered 1 if rij was greater than a specific threshold and zero otherwise. The diagonal elements of the constructed association matrix were also set to zero. The negative correlation values are replaced by zero in the above procedure. Although we lose some information regarding the negative correlations, this procedure is common for binary network analysis of structural brain networks (Bernhardt et al., 2011; Fan et al., 2011). Constructing weighted networks would be more informative but there are still some methodological challenges to analyze and compare weighted and directed networks (Rubinov and Sporns, 2011).
The resultant adjacency matrix represented a binary undirected graph G in which regions i and j were connected if gij was unity. Therefore, a graph was constructed with N=86 nodes (ROIs), with a network degree of E equal to number of edges (links), and a network density (cost) of D=E/[(N×(N–1)]/2] representing the fraction of present connections to all possible connections. It has been shown that thresholding the association matrices of different groups at an absolute threshold results in networks with different number of nodes (and degrees) that influences network measures and reduces interpretation of between-group results (van Wijk et al., 2010). Therefore, based on previous studies (Bassett et al., 2008; Bernhardt et al., 2011; He et al., 2008; Hosseini et al., 2012a, 2012b) two approaches were implemented for thresholding the constructed association matrices: (1) at a minimum network density in which the networks of both groups were not fragmented, and (2) at a range of network densities and comparing the network topologies across that range. For the latter, we thresholded the constructed association matrices at a range of network densities (Dmin: 0.01: 0.45) and compared the network topologies across that range. For densities above 0.45, the graphs became increasingly random (small-world index<1.2) and connections above this density are less likely biological for anatomical networks (Kaiser and Hilgetag, 2006).
Global network measures
Small-world network is an architecture that is simultaneously highly segregated and integrated (Bassett and Bullmore, 2006). Segregation reflects the ability of a network in processing information locally while integration characterizes the ability of a network in processing information globally. Therefore, a small-world network reflects an architecture with optimal balance between local and global information processing. The small-worldness of a complex network is identified by two key metrics: the clustering coefficient C and the characteristic path length L of the network. The clustering coefficient of a node is a measure of the number of edges that exist between its nearest neighbors. The clustering coefficient of a network is the average of clustering coefficients across nodes and is a measure of network segregation. The clustering coefficient thus reflects the overall specialization of a network in information processing. The characteristic path length of a network is the average shortest path length between all pairs of nodes in the network and is the most commonly used measure of network integration (Rubinov and Sporns, 2010). The characteristic path length thus measures the ability of a network in distributed information processing. To evaluate the topology of the brain network, these parameters must be compared to the corresponding mean values of a random graph with the same number of nodes and edges and same degree distribution as the network of interest (Maslov and Sneppen, 2002). Thus, we obtained the small-worldness index of a network as S=[C/Crand]/[L/Lrand] where Crand and Lrand are the mean clustering coefficient and the characteristic path length of 20 random networks (Bassett and Bullmore, 2006; Hosseini et al., 2012b). In a small-world network, the clustering coefficient is significantly higher than that of random networks (C/Crand ratio greater than 1) while the characteristic path length is comparable to random networks (L/Lrand ratio close to 1).
Regional network measures
Since dyslexia is a neurological condition, which might affect a specific regional network, we also investigated the nodal characteristics of the constructed structural networks to identify differences in regional topological parameters between groups. Nodal betweenness centrality and nodal degree were calculated for each of the anatomical ROIs. Nodal betweenness centrality is defined as the fraction of all shortest paths in the network that pass through a given node and is used to detect important anatomical or functional connections. Nodes that bridge disparate parts of the network have a high betweenness centrality (Rubinov and Sporns, 2010). On the other hand, nodal degree is defined as the number of connections that a node has with the rest of the network and is considered a measure of interaction of a node, structurally or functionally, with the network. The quantified nodal degree and betweenness were normalized by the mean network degree and betweenness, respectively, and were then compared between groups (Bernhardt et al., 2011; Hosseini et al., 2012a, 2012b).
Network hubs
Hubs are the most globally connected regions in the brain and are essential for coordinating brain functions through their connectivity with numerous brain regions (Cole et al., 2010). Hubs are not only considered as important regulators of information flow but also play a key role in network resilience to brain injury (Rubinov and Sporns, 2010). We considered a node as a hub if its nodal betweenness centrality was at least 1 SD higher than mean network betweenness centrality (Bassett et al., 2008).
Between-group comparison and statistical analysis
A non-parametric permutation test with 1000 repetitions was used to test the statistical significance of the between-group differences in global and regional network measures (Bassett et al., 2008; He et al., 2008; Hosseini et al., 2012a, 2012b). In each repetition, the residuals for each participant were randomly reassigned to one of the FH+ and FH− groups so that each randomized group had the same number of subjects as the original groups. Then, we obtained an association matrix for each randomized group. The association matrices were then thresholded at a range of network densities that led to a binary adjacency matrix at each threshold. The network measures were then calculated for all the binary adjacency matrices at each density. The differences in network measures between randomized groups were then calculated resulting in a permutation distribution of difference under the null hypothesis. The actual between-group difference in network measures was then placed in the corresponding permutation distribution and a two-tailed p-value was calculated based on its percentile position (Bernhardt et al., 2011). The employed non-parametric permutation test inherently accounts for multiple comparisons (p<0.05) (Nichols and Hayasaka, 2003; Nichols and Holmes, 2001). Brain Connectivity Toolbox (Rubinov and Sporns, 2010) was used for quantification of network measures and our in-house Graph Analysis Toolbox (Hosseini et al., 2012b) was used for comparing the structural networks between groups.
It should be noted that we could not statistically explore the relationship between cognitive–behavioral measures and the extracted network properties. This is because the extracted network measures using T1 structural MRIs can only be generated per group and not on an individual basis. Therefore, we could not perform analysis such as correlations to examine relationships between network properties and cognitive-behavioral outcomes. Future studies using different MRI measures such as high resolution diffusion weighted imaging would circumvent this issue.
Results
Within-group global network measures
The minimum network density in which the networks of both groups were not fragmented was 0.080 for surface area network and 0.074 for thickness network. The association matrices for each group and corresponding binary adjacency matrices thresholded at the minimum density are shown in Fig. S1.
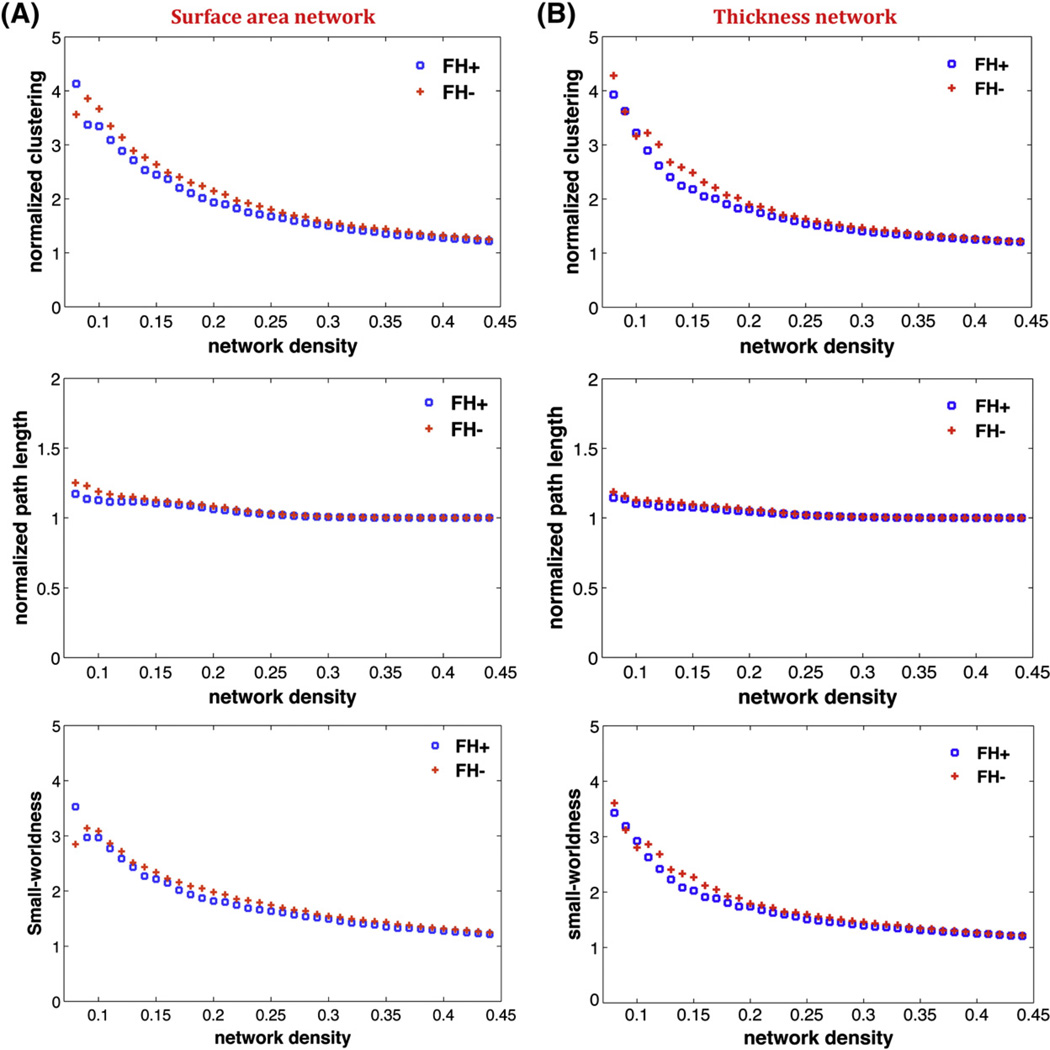
In order to investigate changes in the network topology as a function of network density, we also thresholded the constructed association matrices at a range of network densities (0.01: 0.01: 0.45). For densities above 0.45 the graphs become increasingly random (small-world index <1.2). Additionally, for anatomical networks, connections above this density are less likely biological (Kaiser and Hilgetag, 2006). Changes in global network measures as a function of network cost are shown in Fig. 1. Both the surface area and thickness networks in both groups follow a small-world organization across the range of densities; i.e. normalized characteristic path length (a measure of integration)of the networks was close to one while the normalized clustering coefficient (a measure of segregation) was higher than one. This pattern results in a small-world index of higher than one across the range of network densities.
Fig. 1.
Changes in global network measures as a function of network density. A) Normalized clustering (top), normalized path length (middle), and small-world index (bottom) for surface area network. B) Normalized clustering (top), normalized path length (middle), and small-world index (bottom) for thickness network.
Between-group differences in global network measures
We compared the networks thresholded at the minimum density. For the surface area network, there were no significant differences between groups in global network measures including normalized clustering coefficient (p=0.31), normalized path length (p=0.20), and small-world index (p=0.12). Similarly, for the thickness network, no significant between-group differences were found in normalized clustering coefficient (p=0.31), normalized path length (p=0.40), and small-world index (p=0.21).
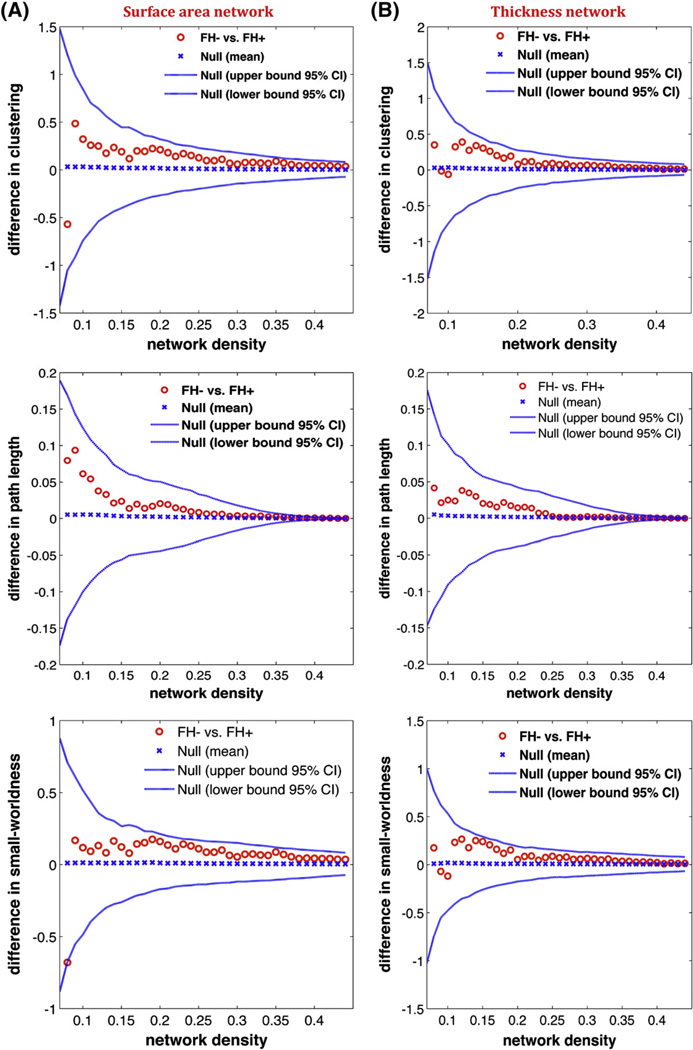
We also examined the between-group differences in network measures across a range of densities (Fig. 2) for both thickness and surface area networks. The results showed no significant difference in global network measures between groups across the range of densities.
Fig. 2.
Between-group differences in global network measures as a function of network density. A) The 95% confidence intervals and between-group differences in normalized clustering (top), normalized path length (middle), and small-world index (bottom) for surface area network. B) The 95% confidence intervals and between-group differences in normalized clustering (top), normalized path length (middle), and small-world index (bottom) for thickness network.
Between-group differences in regional network measures
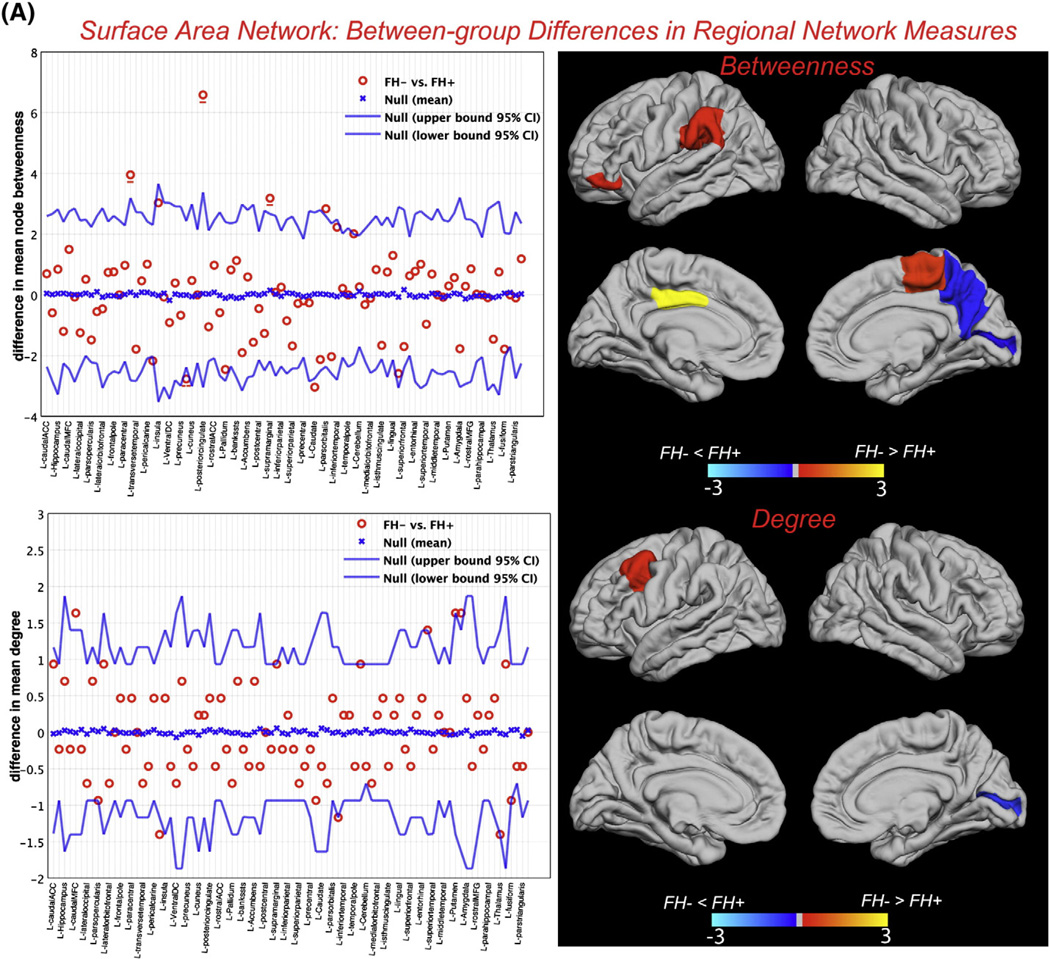
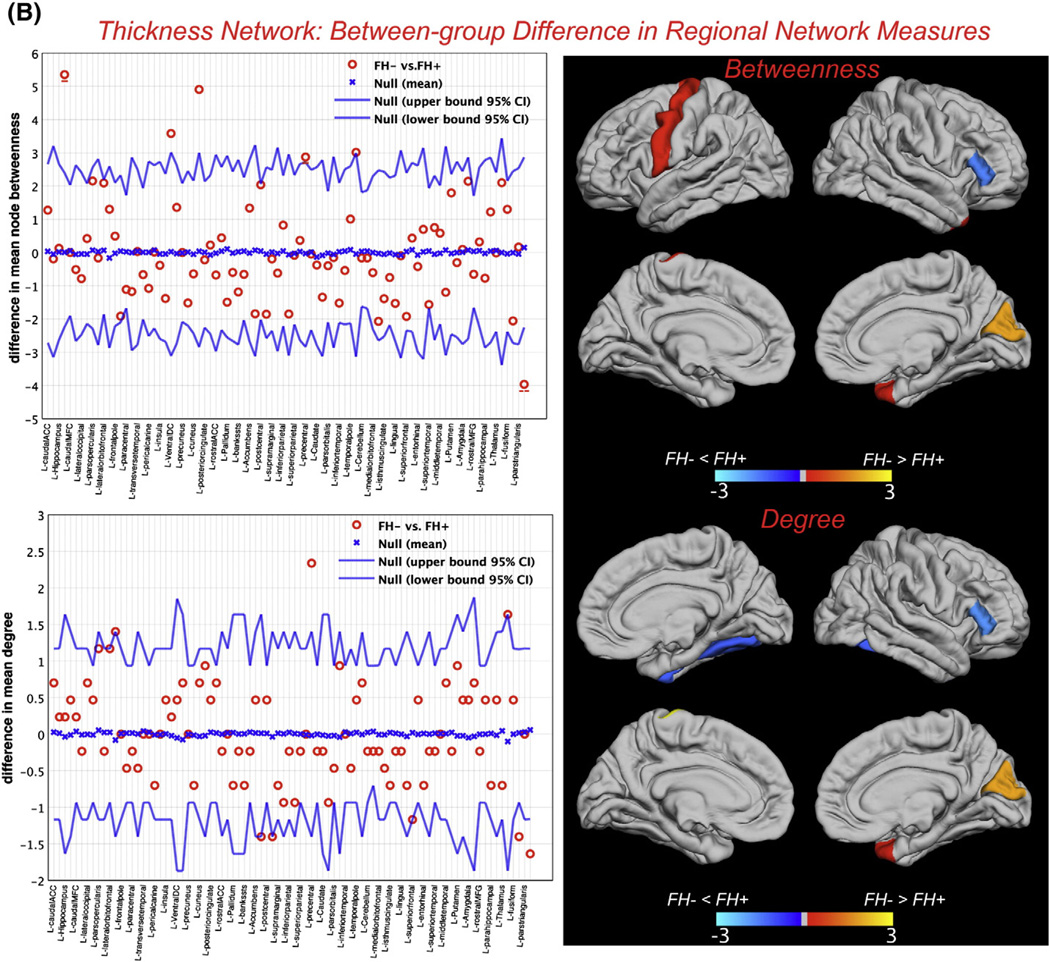
Between-group differences in regional network measures (for networks thresholded at minimum density in which the networks of both groups were not fragmented) including nodal betweenness and nodal degree are shown in Fig. 3. For the surface area network, a number of reading-related regions showed significantly lower betweenness and/or degree in FH+ network including left supramarginal gyrus, left pars orbitalis, and left caudal middle frontal gyrus (Table 2). For the thickness network, brain regions including right pars triangularis and right fusiform gyrus showed significantly higher betweenness and/or degree in FH+ network (Table 3).
Fig. 3.
Between-group differences in regional network topology. A) Between-group differences in nodal betweenness centrality (top) and nodal degree (bottom) for surface area along with the 95% confidence interval of the mean difference. B) Between-group differences in nodal betweenness centrality (top) and nodal degree (bottom) for thickness network along with the 95% confidence interval of the mean difference. For clarity, only regions in the left-hemisphere are labeled and the right hemisphere regions are shown immediately below/to the right of their corresponding left-hemisphere regions and left unlabeled. The color bar represents log(1/p-value). Yellow-color identifies the regions that have significantly higher nodal betweenness/degree in FH− compared with FH+ while blue-color identifies regions with significantly higher nodal betweenness/degree in FH+ compared to FH−. In the betweenness graphs, the hub regions that were significantly different between groups are underscored by solid (FH− hubs) and dashed (FH+ hubs) lines. Specifically, for the surface area network, left posterior cingulate, supramarginal gyrus and paracentral lobule were the FH− hubs that also showed significantly higher betweenness compared with FH+ while the right precuneus was the FH+ hub region that also showed significantly higher betweenness compared with FH−. For the thickness network, right hippocampus and left precentral gyrus were the FH− hubs that also showed significantly higher betweenness compared with FH+ while the right pars triangularis was the FH+ hub region that also showed significantly higher betweenness compared with FH−.
Table 2.
Between-group differences in normalized nodal degree and nodal betweenness centrality for the surface area network.
| Betweenness |
Degree |
||||||
|---|---|---|---|---|---|---|---|
| FH− >FH+ | FH+ | FH− | P | FH− >FH+ | FH+ | FH− | P |
| L pars orbitalis | 0 | 2.83 | 0.043 | R putamen | 1.17 | 2.80 | 0.039 |
| L posterior cingulate | 0.33 | 6.92 | 0.002 | L caudal middle frontal | 0.70 | 2.34 | 0.036 |
| L suprmarginal gyrusa | 0 | 3.18 | 0.038 | ||||
| R paracentral lobule | 0.59 | 4.54 | 0.030 | ||||
| FH + > FH − | FH+ | FH− | P | FH + > FH − | |||
| L caudate | 3.42 | 0.38 | 0.040 | R pericalcarine | 1.87 | 0.47 | 0.027 |
| R pericalcalrine | 2.30 | 0.13 | 0.042 | ||||
| R precuneus | 2.77 | 0 | 0.035 | ||||
L: left, R: right; the average nodal betweenness (degree) was 165.1(6.8) in FH+ and 181.2(6.8) in FH− surface area networks.
Table 3.
Between-group differences in normalized nodal degree and nodal betweenness centrality for the thickness network.
| Betweenness |
Degree |
||||||
|---|---|---|---|---|---|---|---|
| FH− >FH+ | FH+ | FH− | P | FH− >FH+ | FH+ | FH− | P |
| L ventral DC | 1.10 | 4.68 | 0.036 | L precentral gyrus | 0.47 | 2.80 | 0.002 |
| R hippocampus | 1.01 | 6.36 | <0.001 | ||||
| L precentral gyrus | 0.97 | 3.84 | 0.043 | ||||
| R cuneus | 0.039 | 4.94 | 0.007 | ||||
| R temporal pole | 0.021 | 3.04 | 0.049 | ||||
| FH + > FH − | FH+ | FH− | P | FH + > FH − | |||
| R pars triangularis | 3.97 | 0 | 0.010 | R fusiform gyrus | 1.40 | 0 | 0.016 |
| R pars triangularis | 1.64 | 0 | 0.008 | ||||
L: left, R: right; the average nodal betweenness (degree) was 171.2(6.33) in FH+ and 156.2(6.33) in FH− thickness networks.
Network hubs
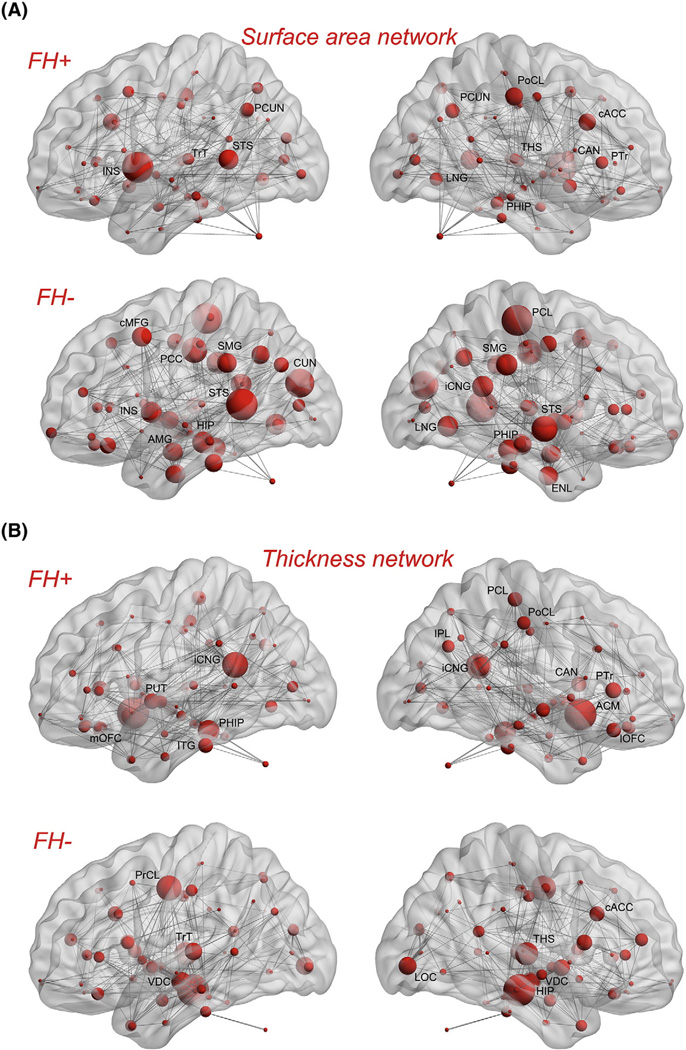
Network hubs were also identified for each group separately (Table 4). For the surface area network, several hubs were found for the FH+ network including right thalamus, left superior temporal sulcus, bilateral precuneus, right caudal anterior cingulate, and right lingual gyrus. Hubs in the FH− network included bilateral superior temporal sulcus, left caudal middle frontal, left posterior cingulate, bilateral supramarginal gyrus, and left insula. For the thickness network, FH+ network hubs included left inferior temporal, bilateral isthmus cingulate, right inferior parietal, and right parstriangularis. FH− network hubs comprised of bilateral ventral diencephalon, right thalamus, right hippocampus, left transverse temporal, and right caudal anterior cingulate. Fig. 4 shows the hubs on groups' network layout mapped on ICBM152 surface template. The identified hubs in the FH− group are consistent with the results of previous graph-theoretical analysis involving healthy subjects (Bassett et al., 2008; He et al., 2008; Hosseini et al., 2012a, 2012b).
Table 4.
List of network hubs for surface-area and thickness networks for each group.
| Surface-area network |
Thickness network |
||
|---|---|---|---|
| FH− network | FH+ network | FH− network | FH+ network |
| L hippocampus | L superior temporal | bilateral diencephalon | L putamen |
| L amygdala | L precuneus | L precentral | R caudate |
| L superior temporal | L transverse temporal | L transverse temporal | R accumbens |
| L caudal middle frontal | L insula | R thalamus | L inferior temporal |
| L cuneus | R thalamus | R hippocampus | L isthmus cingulate |
| L posterior cingulate | R caudate | R anterior cingulate | L medial orbitofrontal |
| L supramarginal | R accumbens | R lateral occipital | L parahippocampal |
| L insula | R anterior cingulate | R inferior parietal | |
| R entorhinal | R lingual | R isthmus cingulate | |
| R isthmus cingulate | R parahippocampal | R lateral orbitofrontal | |
| R lingual | R parstriangularis | R paracentral | |
| R parahippocampal | R postcentral | R pars triangularis | |
| R paracentral | R precuneus | ||
| R superior temporal | |||
| R supramarginal | |||
L: left, R: right.
Fig. 4.
Network hubs: A) Surface area network for FH+ (top) and FH− (bottom) groups. B) Thickness network for FH+ (top) and FH− (bottom) groups. Network hubs are labeled on each scheme separately. The volume of the spheres represents the betweenness centrality of the corresponding brain region. The INS: insula; TrT: transverse temporal; STS: superior temporal sulcus; PCUN: precuneus; LNG: lingual gyrus; THS: thalamus; PoCL: postcentral gyrus; cACC: caudal anterior cingulate; CAN: caudate nucleus; PHIP: parahippocampal gyrus; PTr: pars triangularis; PCL: paracentral lobule; SMG: supramarginal gyrus; iCNG: isthmus cingulate; ENL: entorhinal cortex; cMFG: caudal middle frontal gyrus; PCC: posterior cingulate cortex; CUN: cuneus; HIP: hippocampus; AMG: amygdale; IPL: inferior parietal lobule; ACM: accumbens area; lOFC: lateral orbitofrontal cortex; ITG: inferior temporal gyrus; mOFC: medial orbitofrontal; PUT: putamen; VDC: ventral DC; LOC: lateral occipital; PrCL: precentral gyrus.
Discussion
We investigated differences in structural brain networks between beginning readers with (FH+) and without (FH−) family history for reading difficulties. We constructed separate structural correlation networks based on measures of surface area and thickness that have been shown to be driven by distinct cellular mechanisms that are genetic in their origins (Eyler et al., 2011; Kapellou et al., 2006; Rimol et al., 2010; Sanabria-Diaz et al., 2010). The results showed topological property changes in the FH+ group in several brain regions including those that repeatedly reported to be abnormal in dyslexia (left supramarginal gyrus, left inferior frontal gyrus) as well as other regions that are not often advertised as dyslexia regions but are important contributors in reading (left posterior cingulate, hippocampus, left precentral gyrus). Additionally, alterations in the left-hemisphere reading network were mainly observed for the surface-area network. To our knowledge, this is the first report of altered topological properties of structural correlation networks in children at risk for reading difficulties.
Global network measures
Both the surface area and thickness networks of both the FH+ and FH− groups followed a small-world organization across a wide range of network densities (Fig. 1); both of the networks had a path length identical to random networks while having a clustering coefficient that was higher than that in random networks. Such a network allows for efficient information processing by providing an optimal balance between segregation and integration (He et al., 2008). The results are in line with previous graph analysis studies that have consistently shown a small-world architecture in structural brain networks in healthy individuals (Bassett et al., 2008; Fan et al., 2011; He and Evans, 2010), and in patients with regional brain network deficits such as temporal lobe epilepsy (Bernhardt et al., 2011).
There were no significant between-group differences in global network properties of the surface area and thickness networks. This suggests that the global network segregation and integration is intact in children at familial risk for reading difficulties. These results further corroborate the notion that developmental dyslexia is a reading-specific problem (Ferrer et al., 2010; Tanaka et al., 2011) and is not related to more general cognitive abilities such as intelligence and learning that are associated with global network properties (Li et al., 2009; van den Heuvel et al., 2009).
Regional network measures
We tested differences in centrality and interaction of brain regions by comparing nodal betweenness centrality and nodal degree between FH+ and FH−groups. We observed significant lower betweenness and degree in the left-dominant hemisphere associated with reading in FH+ surface area network. On the other hand, the thickness network results revealed greater interaction and centrality of the right hemispheric regions, often implicated in compensatory processes, in the FH+ compared to the FH− group at an early age.
Surface area network
Betweenness in the left supramarginal gyrus, left pars orbitalis, and left posterior cingulate was significantly lower in the FH+ surface area network. Supramarginal gyrus is part of the reading network associated with analytic processing necessary for the integration of orthographic with phonological features of printed words (Pugh et al., 2000; Simos et al., 2011a). A recent meta-analysis study on reading-related processes reported a consistent hypoactivation in this region in children with developmental dyslexia (Richlan et al., 2011). Lesions in the left supramarginal gyrus have been shown to predict impairments in reading and spelling of words in a cohort lesion study (Philipose et al., 2007). The observed lower betweenness in this region in the FH+ group identifies that the number of shortest paths passing through this region is lower than those for the FH−group. It implies that the information transfer through the left supramarginal gyrus is less efficient in the FH+ group presumably because of lack of proper connections with other reading-related regions. The latter idea is supported by the results of previous functional connectivity analysis that showed impaired connectivity between this region and occipito-temporal regions involved in visual word processing in children with developmental dyslexia (van der Mark et al., 2011).
Left pars orbitalis (Brodmann area 11) was the other region that showed significantly lower betweenness in the FH+ surface area network. This region, though commonly not regarded as part of the reading related network, is part of the inferior frontal circuit (Habib, 2000) associated with phonological processing of single word reading (Turkeltaub et al., 2002) and semantic processing (Vigneau et al., 2005). Activity in this region has been associated with semantic processing during reading (Binder et al., 2003; McDermott et al., 2003; Vigneau et al., 2005). Our observation is in line with the results of a recent meta-analysis on functional neuroimaging studies of reading alphabetic languages that reported significant hypoactivation in the left pars orbitalis in adults with developmental dyslexia (Maisog et al., 2008). It further corroborates previous findings that showed decreased fractional anisotropy of white matter connecting inferior frontal regions and posterior language processing areas in dyslexic adults (Steinbrink et al., 2008) as well as altered effective connectivity between these regions during rhyming judgments of conflicting phonology-orthography trials (Cao et al., 2008).
Another region that showed significantly lower betweenness in FH+ surface area network was the left posterior cingulate. Activation of this region in reading and auditory processing tasks have been linked to language comprehension (Simos et al., 2011b; Stoitsis et al., 2008; Whitney et al., 2009) and attention (Vannest et al., 2009). Hypoactivation of this region in poor-readers has been identified in previous neuroimaging studies on category reading and silent passage reading tasks (Shaywitz et al., 2003; Simos et al., 2011a). Our results suggest that the left posterior cingulate in FH+ surface area network is less efficient in facilitating communication between different brain regions.
Comparison of nodal degree between groups showed significant lower degree in caudal middle frontal gyrus in the FH+ surface area network. This region has long been associated with verbal working memory (Baddeley, 2003) and has recently been shown to play an important role in phonological awareness (Kovelman et al., 2012), an ability that predicts later success in reading in preliterate children (Ziegler and Goswami, 2005). Kovelman et al. (2012) showed that greater demand on phonological awareness caused by an auditory rhyming task resulted in increased activation in the left dorsolateral prefrontal region in typically developing readers but not in children with dyslexia. They argued that children with dyslexia do not engage this region for phonological processing. Our observations both corroborate and extend their findings by suggesting that the impairment in engaging left middle frontal regions in reading tasks in children with reading difficulties might arise from lower overall connectivity of this region with the rest of the network.
Thickness network
For the thickness network, right pars triangularis showed significantly higher degree and betweenness and right fusiform gyrus showed significantly higher degree in the FH+ group. The hyperactivation of these regions in dyslexic readers have been previously reported in several neuroimaging studies on reading and have been associated with a compensatory mechanism. Shaywitz and Shaywitz (2003) reported that during difficult phonologic tasks (nonword rhyming), dyslexic readers engaged the right inferior frontal gyrus in contrast to normal readers. They also reported a negative correlation between reading skill and brain activation in the right fusiform. Activation of these right hemispheric regions has also been reported after experimental intervention in children with reading difficulties (Bach et al., in press; Shaywitz et al., 2004), consistent with our previous study where activation in this region predicted reading outcome in children with dyslexia (Hoeft et al., 2011).
Finally, we also found a number of regions that showed significant differences in betweenness and degree between networks but the exact role of them in developmental dyslexia is still a matter of debate. The higher betweenness in the left caudate nucleus in FH+ surface area network is consistent with the results of previous meta-analysis in adults with dyslexia (Richlan et al., 2011). The observed lower betweenness in other regions including hippocampus, temporal pole in FH+ thickness network and in right putamen in FH− surface area network is also in line with the results of previous studies (Brambati et al., 2009; Casanova et al., 2005; Eliez et al., 2000; Nicolson et al., 1999). Since these structures are part of the implicit learning network, it might reflect differences in nondeclarative aspects of reading between groups (Vicari et al., 2005). Specifically, several studies have reported a significant decrease in left hippocampal volume and/or activity in dyslexia (Casanova et al., 2005; Eliez et al., 2000; Nicolson et al., 1999), emphasizing the important role of this region in word recognition and reading (Papanicolaou et al., 2003). Additionally, left precentral gyrus showed significantly lower degree in FH+ thickness network. Precentral gyrus plays an important role in reading and has been consistently linked to articulation and phonologic retrieval (Carreiras et al., 2007; Houde et al., 2010; Mechelli et al., 2005; Turkeltaub et al., 2003). Our observation is also corroborated by recent evidence that showed significant correlation between resting-state functional connectivity of left precentral gyrus and reading standard scores in children and adults (Koyama et al., 2011).
Together, our results support previous findings that reported disrupted functional and structural connectivity between reading-related regions associated with developmental dyslexia and further suggest that (1) the altered connectivity is evident even in beginning readers at familial risk for reading difficulties and (2) the pattern of altered connectivity is so diffuse that affects the topological properties of reading-related network in structural correlation networks.
Network hub analysis
The results of network hub analysis corroborated the findings of regional topology analysis. While both the FH+ and FH− networks showed a number of common hubs, left supramarginal gyrus was among the highly connected hubs that presents only in FH− surface area network. Conversely, right pars triangularis only presents in the FH+ network hubs. In addition, an interesting finding was a tendency in lateralization of distribution of hubs in the FH+ surface area network (X2(2,28)=1.5, p=0.2). In the FH+ network, a few hubs were identified in the left-hemisphere while hubs were equally distributed between hemispheres in FH− surface area network. This observation supports the results of regional network analysis and suggests that aberrations in reading-related network are more pronounced in the surface-area network.
Surface area vs. thickness networks
Aberrations in topological properties of reading-related network were more pronounced in FH+ surface-area networks while augmentation in connectivity of right-hemispheric regions were observed in FH+ thickness network. While surface area and thickness are both strongly influenced by developmental processes including the genetic/prenatal and postnatal factors, there is some evidence suggesting that the surface area is influenced more by genetic/prenatal factors while thickness showing more changes across life span (Eyler et al., 2011; Im et al., 2008; McGinnis et al., 2011; Rimol et al., 2010; Seo et al., 2007; Wang et al., 2009). While this is still somewhat controversial and speculative, together with previous literature (Black et al., 2012; Frye et al., 2010b), it is tempting to consider these differences reflecting a greater role of genetic/prenatal influence that account for the differences found in this study. Cortical surface area has been shown to be highly heritable (Eyler et al., 2011; Rimol et al., 2010) and increase exuberantly relative to cerebral volume during late fetal development (Kapellou et al., 2006). On the other hand, cortical thickness is more influenced by factors such as aging and disease and changes across the life span (Im et al., 2008; McGinnis et al., 2011; Seo et al., 2007; Wang et al., 2009). Frye et al. (2010b) examined the relationship between phonological skills with cortical thickness and surface area, separately, and reported that only surface area relates to dyslexia. They argued that surface area reflects cortical folding patterns that are mainly determined prenatally. In addition, in our previous study, we also showed that only cortical surface area, and not thickness, relates to severity of maternal history of reading difficulties (Black et al., 2012).
Potential postnatal factors that might influence the results include child's experience due to parental and school environment. Among various prenatal factors (e.g. prenatal environment, genetic and epigenetic phenomena), we speculate that genetic factors may bear on the observed network differences in dyslexia. Previous studies have shown that genetic variation is an important determinant of individual differences in cortical surface area and reported that 89% of variance in surface-area is attributable to genetic factors (Eyler et al., 2011; Panizzon et al., 2009). Specific mutations in humans have been linked to excessive gyrification of the cortex and an increase in the cortical surface area and suggest that the genes that influence surface area are critical to the early growth and development of the brain (Jansen and Andermann, 2005; Panizzon et al., 2009; Piao et al., 2004). Previous studies on dyslexia also support the predominant influence of genetic factors (rather than postnatal factors) in dyslexia. Studies show that parents' reading scores correlate with that of offspring (Torppa et al., 2011; van Bergen et al., 2012). We have also shown that maternal history of reading disability correlated with preliterate children's frontoparietal structures, most likely due to genetic or prenatal environmental influences (Black et al., 2012). While the underlying mechanism of these associations is unclear, genetic liability is thought to play a major role (Naples et al., 2009, 2012), consistent with a meta-analysis that showed high heritability estimates of 41–74% (Grigorenko, 2004). Further support comes from a study in adoptive families that showed lack of parent-offspring relationship in reading (Wadsworth et al., 2002), and animal studies that showed lack of intrauterine transmission of ectopia in rodents (Denenberg et al., 1991, 1992). In rodents, ectopia is often used as a ‘proxy’ for animal models of dyslexia (Kaufmann and Galaburda, 1989). Ectopia has been associated with DYX1C1, a candidate gene for dyslexia that causes neuronal migration abnormalities (Rosen et al., 2007). Another candidate gene, the ROBO1 gene, regulate midline crossing of major nerve tracts and have been associated with abnormal auditory cortex responses in individuals with dyslexia (Lamminmäki et al., 2012). In another recent study, FOXP2, a gene that causes a severe form of language impairment and KIAA0319, another candidate gene, were associated with the left frontal and parietotemporal activation patterns, respectively (Pinel et al., 2012). These studies point to the important role of genetic variation in the neural pathways important for dyslexia, reading and phonological processing. On the other hand, not many studies have shown the influence of prenatal and postnatal environment. In one study, intrauterine serum factors caused rodent offspring's abnormal behavior and brain metabolism that were consistent with the magnocellular theory of dyslexia, implicating the role of prenatal environment in the development of dyslexia (Vincent et al., 2002). Collectively, while still not conclusive, current evidence supports a major genetic influence in the development of dyslexia. Future research is needed to directly test the observed distinctions and to investigate how cortical structure relates to early development and the genotype.
Genetic etiology
Previous studies have identified a number of genetic loci and candidate genes related to dyslexia [see (Poelmans et al., 2011) for a recent review]. DCDC2, ROBO1, DYX1C1 and KIAA0319 are among the candidate genes that have been previously associated with developmental dyslexia. These genes could be directly or indirectly linked to processes involved in neuronal migration and/or directed outgrowth of neuritis/axons and thus suggest that dyslexia is a neuronal migration deficit (Petryshen and Pauls, 2009). While there is some evidence of links to cortical patterning in language related regions (e.g. Meda et al., 2008 for DCDC2), the reports are on regional volumes without differentiation between surface and thickness. MECP2 is one of the main determinants of brain morphology and its mutations are associated with a number of neurodevelopmental disorders such as autism and mild learning disabilities (Shibayama et al., 2004). The relationship between variations in the MECP2 and brain morphology has been shown in primates and human (Belichenko et al., 2008; Kankirawatana et al., 2006). Recent evidence suggests that variations in this gene are associated with cortical surface area but not cortical thickness (Joyner et al., 2009). This allele could be a candidate explaining the dysfunction of surface area network in FH+ group in language-related regions. Previous evidence has shown that variations in MECP2 were found in Klinefelter's Syndrome individuals that often show reading impairment (Vawter et al., 2007). Uchino et al. (2001) also reported a strong correlation between the loci of mutation in the MECP2 gene and language ability in subjects with Rett Syndrome. In addition, variation in this allele has been shown to affect surface area of brain regions specific to reading comprising pars triangularis (Joyner et al., 2009). Future research might shed light on the influence of the MECP2 gene on developmental dyslexia.
Together, our results suggest that topological properties of large-scale structural brain networks could be informative for predicting the risk of reading difficulties in children. However, there are a number of limitations and future work is warranted that overcomes these limitations. First, the demographics of the group show that most of the standard scores are within average range, and hence while half of the children have a family history for reading disabilities, they are not necessarily at risk based on their behavioral characteristics. However, these children might still be at risk for late emerging reading disabilities and we are planning to follow them to a later age. Second, we could not perform correlation analysis between these measures and the network results because of the nature of the underlying data (in that we used structural T1 MRIs that does not allow us to estimate one graph per participant). Recent studies have shown the possibility of constructing such a network at an individual level using high resolution diffusion weighted imaging (Gong et al., 2009; van den Heuvel et al., 2009) which will overcome this limitation. Finally, in our current study, we used the traditional approach to define groups based on overall family history status; it may be interesting to examine the effect of maternal and paternal history on network characteristics separately in future studies in line with our previous work (Black et al., 2012).
Supplementary Material
Acknowledgments
This study was supported by grants from the National Institute of Child Health and Human Development (K23HD054720 to FH), Lucile Packard Foundation for Children's Health (LPFCH), Spectrum Child Health, Clinical and Translational Science Award, and the Dyslexia Foundation to FH, and (P41RR009784 to Dr. Gary Glover) at Stanford University. Funding for JMB was provided by a National Institute of Health (NIH)-sponsored institutional research training grant (T32) (5T32MH019908-17 to Dr. Allan Reiss) at Stanford University.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.neuroimage.2013.01.013.
References
- Bach S, Richardson U, Brandeis D, Martin E, Brem S. Print-specific multimodal brain activation in kindergarten improves prediction of reading skills in second grade. NeuroImage. doi: 10.1016/j.neuroimage.2013.05.062. in press. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working memory: looking back and looking forward. Nat. Rev. Neurosci. 2003;4:829–839. doi: 10.1038/nrn1201. [DOI] [PubMed] [Google Scholar]
- Bassett DS, Bullmore E. Small-world brain networks. Neuroscientist. 2006;12:512–523. doi: 10.1177/1073858406293182. [DOI] [PubMed] [Google Scholar]
- Bassett DS, Bullmore E, Verchinski BA, Mattay VS, Weinberger DR, Meyer-Lindenberg A. Hierarchical organization of human cortical networks in health and schizophrenia. J. Neurosci. 2008;28:9239–9248. doi: 10.1523/JNEUROSCI.1929-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belichenko NP, Belichenko PV, Li HH, Mobley WC, Francke U. Comparative study of brain morphology in Mecp2 mutant mouse models of Rett syndrome. J. Comp. Neurol. 2008;508:184–195. doi: 10.1002/cne.21673. [DOI] [PubMed] [Google Scholar]
- Ben-Yehudah G, Fiez JA. Impact of cerebellar lesions on reading and phonological processing. Ann. N. Y. Acad. Sci. 2008;1145:260–274. doi: 10.1196/annals.1416.015. [DOI] [PubMed] [Google Scholar]
- Bernhardt BC, Worsley KJ, Besson P, Concha L, Lerch JP, Evans AC, Bernasconi N. Mapping limbic network organization in temporal lobe epilepsy using morphometric correlations: insights on the relation between mesiotemporal connectivity and cortical atrophy. NeuroImage. 2008;42:515–524. doi: 10.1016/j.neuroimage.2008.04.261. [DOI] [PubMed] [Google Scholar]
- Bernhardt BC, Chen Z, He Y, Evans AC, Bernasconi N. Graph-theoretical analysis reveals disrupted small-world organization of cortical thickness correlation networks in temporal lobe epilepsy. Cereb. Cortex. 2011;21:2147–2157. doi: 10.1093/cercor/bhq291. [DOI] [PubMed] [Google Scholar]
- Binder JR, McKiernan KA, Parsons ME, Westbury CF, Possing ET, Kaufman JN, Buchanan L. Neural correlates of lexical access during visual word recognition. J. Cogn. Neurosci. 2003;15:372–393. doi: 10.1162/089892903321593108. [DOI] [PubMed] [Google Scholar]
- Black JM, Tanaka H, Stanley L, Nagamine M, Zakerani N, Thurston A, Kesler S, Hulme C, Lyytinen H, Glover GH, Serrone C, Raman MM, Reiss AL, Hoeft F. Maternal history of reading difficulty is associated with reduced language-related gray matter in beginning readers. NeuroImage. 2012;59:3021–3032. doi: 10.1016/j.neuroimage.2011.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein EH, Bradley RH. Socioeconomic Status, Parenting, and Child Development. Mahwah: Lawrence Erlbaum Associates; 2003. [Google Scholar]
- Brambati SM, Ogar J, Neuhaus J, Miller BL, Gorno-Tempini ML. Reading disorders in primary progressive aphasia: a behavioral and neuroimaging study. Neuropsychologia. 2009;47:1893–1900. doi: 10.1016/j.neuropsychologia.2009.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brem S, Bach S, Kucian K, Guttorm TK, Martin E, Lyytinen H, Brandeis D, Richardson U. Brain sensitivity to print emerges when children learn letter-speech sound correspondences. Proc. Natl. Acad. Sci. U. S. A. 2010;107:7939–7944. doi: 10.1073/pnas.0904402107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell B, Bradley R. Home Observation for Measurement of the Environment. Little Rock: University of Arkansas; 1984. [Google Scholar]
- Cao F, Bitan T, Booth JR. Effective brain connectivity in children with reading difficulties during phonological processing. Brain Lang. 2008;107:91–101. doi: 10.1016/j.bandl.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreiras M, Mechelli A, Estevez A, Price K. Brain activation for lexical decision and reading aloud: Two sides of the same coin? J. Cogn. Neurosci. 2007;19(3):433–444. doi: 10.1162/jocn.2007.19.3.433. [DOI] [PubMed] [Google Scholar]
- Casanova MF, Christensen JD, Giedd J, Rumsey JM, Garver DL, Postel GC. Magnetic resonance imaging study of brain asymmetries in dyslexic patients. J. Child Neurol. 2005;20:842–847. doi: 10.1177/08830738050200101401. [DOI] [PubMed] [Google Scholar]
- Chen ZJ, He Y, Rosa-Neto P, Germann J, Evans AC. Revealing modular architecture of human brain structural networks by using cortical thickness from MRI. Cereb. Cortex. 2008;18:2374–2381. doi: 10.1093/cercor/bhn003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheverud JM. Quantitative genetics and developmental constraints on evolution by selection. J. Theor. Biol. 1984;110:155–171. doi: 10.1016/s0022-5193(84)80050-8. [DOI] [PubMed] [Google Scholar]
- Cole MW, Pathak S, Schneider W. Identifying the brain's most globally connected regions. NeuroImage. 2010;49:3132–3148. doi: 10.1016/j.neuroimage.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B. Cortical surface-based analysis: I. Segmentation and surface reconstruction. NeuroImage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Dale AM, Sereno MI. Improved localizadon of cortical activity by combining eeg and meg with mri cortical surface reconstruction: a linear approach. J. Cogn. Neurosci. 1993;5:162–176. doi: 10.1162/jocn.1993.5.2.162. [DOI] [PubMed] [Google Scholar]
- Denenberg VH, Mobraaten LE, Sherman GF, Morrison L, Schrott LM, Waters NS, Rosen GD, et al. Effects of the autoimmune uterine/maternal environment upon cortical ectopias, behavior and autoimmunity. Brain Res. 1991;563(1–2):114–122. doi: 10.1016/0006-8993(91)91522-3. [DOI] [PubMed] [Google Scholar]
- Denenberg VH, Sherman GF, Morrison L, Schrott LM, Waters NS, Rosen GD, Behan PO, et al. Behavior, ectopias and immunity in BD/DB reciprocal crosses. Brain Res. 1992;571(2):323–329. doi: 10.1016/0006-8993(92)90671-u. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire P, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Eckert MA, Leonard CM, Wilke M, Eckert M, Richards T, Richards A, Berninger V. Anatomical signatures of dyslexia in children: unique information from manual and voxel based morphometry brain measures. Cortex. 2005;41:304–315. doi: 10.1016/s0010-9452(08)70268-5. [DOI] [PubMed] [Google Scholar]
- Eliez S, Rumsey JM, Giedd JN, Schmitt JE, Patwardhan AJ, Reiss AL. Morphological alteration of temporal lobe gray matter in dyslexia: an fMRI study. J. Child Psychol. Psychiatry. 2000;41(5):637–644. doi: 10.1111/1469-7610.00650. [DOI] [PubMed] [Google Scholar]
- Eyler LT, Prom-Wormley E, Panizzon MS, Kaup AR, Fennema-Notestine C, Neale MC, Jernigan TL, Fischl B, Franz CE, Lyons MJ, Grant M, Stevens A, Pacheco J, Perry ME, Schmitt JE, Seidman LJ, Thermenos HW, Tsuang MT, Chen CH, Thompson WK, Jak A, Dale AM, Kremen WS. Genetic and environmental contributions to regional cortical surface area in humans: a magnetic resonance imaging twin study. Cereb. Cortex. 2011;21:2313–2321. doi: 10.1093/cercor/bhr013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Shi F, Smith JK, Lin W, Gilmore JH, Shen D. Brain anatomical networks in early human brain development. NeuroImage. 2011;54:1862–1871. doi: 10.1016/j.neuroimage.2010.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer E, Shaywitz BA, Holahan JM, Marchione K, Shaywitz SE. Uncoupling of reading and IQ over time: empirical evidence for a definition of dyslexia. Psychol. Sci. 2010;21:93–101. doi: 10.1177/0956797609354084. [DOI] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc. Natl. Acad. Sci. U. S. A. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis II: inflation, flattening, and a surface-based coordinate system. NeuroImage. 1999a;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RBH, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum. Brain Mapp. 1999b;8:272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Liu A, Dale AM. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans. Med. Imaging. 2001;20:70–80. doi: 10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, van der Kouwe AJW, Makris N, Segonne F, Quinn BT, Dale AM. Sequence-independent segmentation of magnetic resonance images. NeuroImage. 2004a;23:S69–S84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C. Automatically parcellating the human cerebral cortex. Cereb. Cortex. 2004b;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Frye RE, Liederman J, Hasan KM, Lincoln A, Malmberg B, McLean J, Papanicolaou A. Diffusion tensor quantification of the relations between microstructural and macrostructural indices of white matter and reading. Hum. Brain Mapp. 2010a;32:1220–1235. doi: 10.1002/hbm.21103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye RE, Liederman J, Malmberg B, McLean J, Strickland D, Beauchamp MS. Surface area accounts for the relation of gray matter volume to reading-related skills and history of dyslexia. Cereb. Cortex. 2010b;20:2625–2635. doi: 10.1093/cercor/bhq010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye RE, Wu MH, Liederman J, Fisher JM. Greater pre-stimulus effective connectivity from the left inferior frontal area to other areas is associated with better phonological decoding in dyslexic. Front. Syst. Neurosci. 2010c;4:1–13. doi: 10.3389/fnsys.2010.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong G, He Y, Concha L, Lebel C, Gross DW, Evans AC, Beaulie C. Mapping anatomical connectivity patterns of human cerebral cortex using in vivo diffusion tensor imaging tractography. Cereb. Cortex. 2009;19:524–536. doi: 10.1093/cercor/bhn102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigorenko EL. Genetic bases of developmental dyslexia: a capsule review of heritability estimates. Enfance. 2004;56(3):273. [Google Scholar]
- Habib M. The neurological basis of developmental dyslexia. Brain. 2000;123:2373–2399. doi: 10.1093/brain/123.12.2373. [DOI] [PubMed] [Google Scholar]
- Han X, Jovicich J, Salat D, van der Kouwe A, Quinn B, Czanner S, Busa E, Pacheco J, Albert M, Killiany R, Maguire P, Rosas D, Makris N, Dale A, Dickerson D, Fisch B. Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. NeuroImage. 2006;32:180–194. doi: 10.1016/j.neuroimage.2006.02.051. [DOI] [PubMed] [Google Scholar]
- He Y, Evans A. Graph theoretical modeling of brain connectivity. Curr. Opin. Neurol. 2010;23:341–350. doi: 10.1097/WCO.0b013e32833aa567. [DOI] [PubMed] [Google Scholar]
- He Y, Chen ZJ, Evans AC. Small-world anatomical networks in the human brain revealed by cortical thickness from MRI. Cereb. Cortex. 2007;17:2407–2419. doi: 10.1093/cercor/bhl149. [DOI] [PubMed] [Google Scholar]
- He Y, Chen Z, Evans A. Structural insights into aberrant topological patterns of large-scale cortical networks in Alzheimer's disease. J. Neurosci. 2008;28:4756–4766. doi: 10.1523/JNEUROSCI.0141-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Dagher A, Chen Z, Charil A, Zijdenbos A, Worsley K, Evans A. Impaired small-world efficiency in structural cortical networks in multiple sclerosis associated with white matter lesion load. Brain. 2009;132:3366–3379. doi: 10.1093/brain/awp089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeft F, Hernandez A, McMillon G, Taylor-Hill H, Martindale JL, Meyler A, Keller TA, Siok WT, Deutsch GK, Just MA, Whitfield-Gabrieli S, Gabrieli JD. Neural basis of dyslexia: a comparison between dyslexic and nondyslexic children equated for reading ability. J. Neurosci. 2006;26:10700–10708. doi: 10.1523/JNEUROSCI.4931-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeft F, Meyler A, Hernandez A, Juel C, Taylor-Hill H, Martindale JL, McMillon G, Kolchugina G, Black JM, Faizi A, Deutsch GK, Siok WT, Reiss AL, Whitfield-Gabrieli S, Gabrieli JDE. Functional and morphometric brain dissociation between dyslexia and reading ability. Proc. Natl. Acad. Sci. U. S. A. 2007;104:4234–4239. doi: 10.1073/pnas.0609399104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeft F, McCandliss BD, Black JM, Gantman A, Zakerani N, Hulme C, Lyytinen H, Whitfield-Gabrieli S, Glover GH, Reiss AL, Gabrieli JDE. Neural systems predicting long-term outcome in dyslexia. Proc. Natl. Acad. Sci. U. S. A. 2011;108:361–366. doi: 10.1073/pnas.1008950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz B, Rumsey JM, Donohue BC. Functional connectivity of the angular gyrus in normal reading and dyslexia. Proc. Natl. Acad. Sci. U. S. A. 1998;95:8939–8944. doi: 10.1073/pnas.95.15.8939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini SMH, Koovakkattu D, Kesler SR. Altered small-world properties of gray matter networks in breast cancer. BMC Neurol. 2012a;12:28. doi: 10.1186/1471-2377-12-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini SMH, Hoeft F, Kesler SR. GAT: a graph-theoretical analysis toolbox for analyzing between-group differences in large-scale structural and functional brain networks. PLoS One. 2012b;7(7):e40709. doi: 10.1371/journal.pone.0040709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houde O, Rossi S, Lubin A, Joliot M. Mapping numerical processing, reading, and executive functiona in the developing brain.: an fMRI meta-analsyis of 52 stduies including 842 children. Dev. Sci. 2010;13(6):876–885. doi: 10.1111/j.1467-7687.2009.00938.x. [DOI] [PubMed] [Google Scholar]
- Im K, Lee JM, Won Seo S, Hyung Kim S, Kim SI, Na DL. Sulcal morphology changes and their relationship with cortical thickness and gyral white matter volume in mild cognitive impairment and Alzheimer's disease. NeuroImage. 2008;43:103–113. doi: 10.1016/j.neuroimage.2008.07.016. [DOI] [PubMed] [Google Scholar]
- Jansen A, Andermann E. Genetics of the polymicrogyria syndromes. J. Med. Genet. 2005;42:369–378. doi: 10.1136/jmg.2004.023952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyner AH, Roddey JC, Bloss CS, Bakken TE, Rimol LM, Melle I, Agartz I, Djurovic S, Topol EJ, Schork NJ, Andreassen OA, Dale AM. A common MECP2 haplotype associates with reduced cortical surface area in humans in two independent populations. Proc. Natl. Acad. Sci. U. S. A. 2009;106:15483–15488. doi: 10.1073/pnas.0901866106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser M, Hilgetag CC. Nonoptimal component placement, but short processing paths, due to long-distance projections in neural systems. PLoS Comput. Biol. 2006;2:e95. doi: 10.1371/journal.pcbi.0020095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kankirawatana P, Leonard H, Ellaway C, Scurlock J, Mansour A, Makris CM, Dure LS, Friez M, Lane J, Kiraly-Borri C, Fabian V, Davis M, Jackson J, Christodoulou J, Kaufmann WE, Ravine D, Percy AK. Early progressive encephalopathy in boys and MECP2 mutations. Neurology. 2006;67:164–166. doi: 10.1212/01.wnl.0000223318.28938.45. [DOI] [PubMed] [Google Scholar]
- Kapellou O, Counsell SJ, Kennea N, Dyet L, Saeed N, Stark J, Maalouf E, Duggan P, Ajayi-Obe M, Hajnal J, Allsop JM, Boardman J, Rutherford MA, Cowan F, Edwards AD. Abnormal cortical development after premature birth shown by altered allometric scaling of brain growth. PLoS Med. 2006;3:e265. doi: 10.1371/journal.pmed.0030265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann WE, Galaburda AM. Cerebrocortical microdysgenesis in neurologically normal subjects: a histopathologic study. Neurology. 1989;39:238–244. doi: 10.1212/wnl.39.2.238. [DOI] [PubMed] [Google Scholar]
- Kovelman I, Norton ES, Christodoulou JA, Gaab N, Lieberman DA, Triantafyllou C, Wolf M, Whitfield-Gabrieli S, Gabrieli JD. Brain Basis of Phonological Awareness for Spoken Language in Children and Its Disruption in Dyslexia. Cereb. Cortex. 2012;22:754–764. doi: 10.1093/cercor/bhr094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama MS, Martino AD, Zuo X, Kelly C, Mennes M, Jutagir DR, Castellons FX, Milham MP. Resting-state functional connectivity indexes reading cometence in children and adults. J. Neurosci. 2011;31(23):8617–8624. doi: 10.1523/JNEUROSCI.4865-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuperberg GR, Broome MR, McGuire PK, David AS, Eddy M, Ozawa F, Goff D, West WC, Williams SC, van der Kouwe AJ, Salat DH, Dale AM, Fischl B. Regionally localized thinning of the cerebral cortex in schizophrenia. Arch. Gen. Psychiatry. 2003;60:878–888. doi: 10.1001/archpsyc.60.9.878. [DOI] [PubMed] [Google Scholar]
- Lamminmäki S, Massinen S, Nopola-Hemmi J, Kere J, Hari R. Human ROBO1 regulates interaural interaction in auditory pathways. J. Neurosci. 2012;32(3):966–971. doi: 10.1523/JNEUROSCI.4007-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefly DL, Pennington BF. Reliability and validity of the adult reading history questionnaire. J. Learn. Disabil. 2000;33:286–296. doi: 10.1177/002221940003300306. [DOI] [PubMed] [Google Scholar]
- Lerch JP, Worsley K, Shaw WP, Greenstein DK, Lenroot RK, Giedd J, Evans AC. Mapping anatomical correlations across cerebral cortex (MACACC) using cortical thickness from MRI. NeuroImage. 2006;31:993–1003. doi: 10.1016/j.neuroimage.2006.01.042. [DOI] [PubMed] [Google Scholar]
- Li Y, Liu Y, Li J, Qin W, Li K, Yu C, Jiang T. Brain anatomical network and intelligence. PLoS Comput. Biol. 2009;5:e1000395. doi: 10.1371/journal.pcbi.1000395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyytinen H, Erskine J, Tolvanen A, Torppa M, Poikkeus A, Lyytinen P. Trajectories of reading development: a follow-up from birth to school age of children with and without risk for dyslexia. Merrill-Palmer Q. 2006;52:514–546. [Google Scholar]
- Lyytinen H, Erskine J, Ahonen T, Aro M, Eklund K, Guttorm T, Hintikka S, Hamalainen J, Ketonen R, Laakso ML, Leppanen PHT, Lyytinen P, Poikkeus AM, Puolakanaho A, Richardson U, Salmi P, Tolvanen A, Torppa M, Viholainen H. Early identification and prevention of dyslexia: results from a prospective follow-up study of children at familial risk for dyslexia. In: Reid G, Fawcett AJ, Manis F, Siegel LS, editors. The SAGE Handbook of Dyslexia. Thousand Oaks: SAGE Publications Ltd.; 2008. pp. 121–146. [Google Scholar]
- Maisog JM, Einbinder ER, Flowers DL, Turkeltaub PE, Edena GF. A meta-analysis of functional neuroimaging studies of dyslexia. Ann. N. Y. Acad. Sci. 2008;1145:237–259. doi: 10.1196/annals.1416.024. [DOI] [PubMed] [Google Scholar]
- Maslov S, Sneppen K. Specificity and stability in topology of protein networks. Science. 2002;296:910–913. doi: 10.1126/science.1065103. [DOI] [PubMed] [Google Scholar]
- Maurer U, Bucher K, Brem S, Brandeis D. Altered responses to tone and phoneme mismatch in kindergartners at familial dyslexia risk. Neuroreport. 2003;14:2245–2250. doi: 10.1097/00001756-200312020-00022. [DOI] [PubMed] [Google Scholar]
- Maurer U, Brem S, Bucher K, Kranz F, Benz R, Steinhausen C, Brandeis D. Impaired tuning of a fast occipito-temporal response for print in dyslexic children learning to read. Brain. 2007;130:3200–3210. doi: 10.1093/brain/awm193. [DOI] [PubMed] [Google Scholar]
- Maurer U, Bucher K, Brem S, Benz R, Kranz F, Schulz E, van der Mark S, Steinhausen HC, Brandeis D. Neurophysiology in preschool improves behavioral prediction of reading ability throughout primary school. Biol. Psychiatry. 2009;66:341–348. doi: 10.1016/j.biopsych.2009.02.031. [DOI] [PubMed] [Google Scholar]
- McDermott KB, Petersen SE, Watson JM, Ojemann G. A procedure for identifying regions preferentially activated by attention to semantic and phonological relations using functional magnetic resonance imaging. Neuropsychologia. 2003;41:293–303. doi: 10.1016/s0028-3932(02)00162-8. [DOI] [PubMed] [Google Scholar]
- McGinnis SM, Brickhouse M, Pascual B, Dickerson BC. Age-related changes in the thickness of cortical zones in humans. Brain Topogr. 2011;24:279–291. doi: 10.1007/s10548-011-0198-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechelli A, Crinion J, Long S, Friston KJ, Lambon-Ralph MA, Patterson K, et al. Dissociating reading processes on the basis of neurona interactions. J. Cogn. Neurosci. 2005;17:1753–1756. doi: 10.1162/089892905774589190. [DOI] [PubMed] [Google Scholar]
- Meda SA, Gelernter J, Gruen JR, Calhoun VD, Meng H, Cope NA, Pearlson G. Polymorphism of DCDC2 reveals differences in cortical morphology of healthy individuals – a preliminary voxel based morphometry study. Brain Imaging Behav. 2008;2:21–26. doi: 10.1007/s11682-007-9012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naples AJ, Chang JT, Katz L, Grigorenko EL. Same or different? Insights into the etiology of phonological awareness and rapid naming. Biol. Psychol. 2009;80(2):226–239. doi: 10.1016/j.biopsycho.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naples A, Katz L, Grigorenko EL. Lexical decision as an endophenotype for reading comprehension: an exploration of an association. Dev. Psychopathol. 2012;24:1345–1360. doi: 10.1017/S0954579412000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols TE, Hayasaka S. Controlling the family wise error rate in functional neuroimaging: a comparative review. Stat. Methods Med. Res. 2003;12:419–446. doi: 10.1191/0962280203sm341ra. [DOI] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum. Brain Mapp. 2001;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson RI, Fawcett AJ, Berry EL, Jenkins IH, Dean P, Brooks DJ. Association of abnormal cerebellar activation with motor learning difficulties in dyslexic adults. Lancet. 1999;353(9165):1662–1667. doi: 10.1016/S0140-6736(98)09165-X. [DOI] [PubMed] [Google Scholar]
- Noble KG, Wolmetz ME, Ochs LG, Farah MJ, McCandliss BD. Brain–behavior relationships in reading acquisition are modulated by socioeconomic factors. Dev. Sci. 2006;9:642–654. doi: 10.1111/j.1467-7687.2006.00542.x. [DOI] [PubMed] [Google Scholar]
- Odegard TN, Farris EA, Ring J, McColl R, Black J. Brain connectivity in non-reading impaired children and children diagnosed with developmental dyslexia. Neuropsychologia. 2009;47:1972–1977. doi: 10.1016/j.neuropsychologia.2009.03.009. [DOI] [PubMed] [Google Scholar]
- Panizzon MS, Fennema-Notestine C, Eyler LT, Jernigan TL, Prom-Wormley E, Neale M, Jacobson K, Lyons MJ, Grant MD, Franz CE, et al. Distinct genetic influences on cortical surface area and cortical thickness. Cereb. Cortex. 2009;19:2728–2735. doi: 10.1093/cercor/bhp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papanicolaou AC, Panagiotis GS, Breier JI, Fltcher JM, Foorman BR, Francis D, Castillo EM, Davis RN. Brain mechanisms for reading in children with and wihout dyslexia: a review of studies of normal development and plasticity. Dev. Neuropsychol. 2003;24:593–612. doi: 10.1080/87565641.2003.9651912. [DOI] [PubMed] [Google Scholar]
- Pennington BF, Lefly DL. Early reading development in children at family risk for dyslexia. Child Dev. 2001;72:816–833. doi: 10.1111/1467-8624.00317. [DOI] [PubMed] [Google Scholar]
- Petryshen TL, Pauls DL. The genetics of reading disability. Curr. Psychiatry Rep. 2009;11:149–155. doi: 10.1007/s11920-009-0023-z. [DOI] [PubMed] [Google Scholar]
- Philipose LE, Gottesman RF, Newhart M, Kleinman JT, Herskovits EH, Pawlak MA, Marsh EB, Davis C, Heidler-Gary J, Hillis AE. Neural regions essential for reading and spelling of words and pseudowords. Ann. Neurol. 2007;62:481–492. doi: 10.1002/ana.21182. [DOI] [PubMed] [Google Scholar]
- Piao X, Hill RS, Bodell A, Chang BS, Basel-Vanagaite L, Straussberg R, Dobyns WB, Qasrawi B, Winter RM, Innes AM, et al. G protein-coupled receptor-depndent development of human frontal cortex. Science. 2004;303:2033–2036. doi: 10.1126/science.1092780. [DOI] [PubMed] [Google Scholar]
- Pinel P, Fauchereau F, Moreno A, Barbot A, Lathrop M, Zelenika D, Le Bihan D, et al. Genetic variants of FOXP2 and KIAA0319/TTRAP/THEM2 locus are associated with altered brain activation in distinct language-related regions. J. Neurosci. 2012;32(3):817–825. doi: 10.1523/JNEUROSCI.5996-10.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poelmans G, Buitelaar JK, Pauls DL, Franke B. A theoretical molecular network for dyslexia: integrating available genetic findinigs. Mol. Psychiatry. 2011;16(4):365–382. doi: 10.1038/mp.2010.105. [DOI] [PubMed] [Google Scholar]
- Pugh KR, Mencl WE, Jenner AR, Katz L, Frost SJ, Lee JR, Shaywitz SE, Shaywitz BA. Functional neuroimaging studies of reading and reading disability (developmental dyslexia) Ment. Retard. Dev. Disabil. Res. Rev. 2000;6:207–213. doi: 10.1002/1098-2779(2000)6:3<207::AID-MRDD8>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Puolakanaho A, Ahonen T, Aro M, Eklund E, Leppanen PHT, Poikkeus AM, Tolvanen A, Torppa M, Lyytinen H. Very early phonological and language skills: estimating individual risk of reading disability. J. Child Psychol. Psychiatry. 2007;48(9):923–931. doi: 10.1111/j.1469-7610.2007.01763.x. [DOI] [PubMed] [Google Scholar]
- Raschle NM, Chang M, Gaab N. Structural brain alterations associated with dyslexia predate reading onset. NeuroImage. 2011;57:742–749. doi: 10.1016/j.neuroimage.2010.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschle NM, Zuk J, Gaab N. Functional characteristics of developmental dyslexi in left-hemisphereic posterior brain regions predate reading onset. PNAS. 2012;109(6):2156–2161. doi: 10.1073/pnas.1107721109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richlan F, Kronbichler M, Wimmer H. Functional abnormalities in the dyslexic brain: a quantitative meta-analysis of neuroimaging studies. Hum. Brain Mapp. 2009;30:3299–3308. doi: 10.1002/hbm.20752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richlan F, Kronbichler M, Wimmer H. Meta-analyzing brain dysfunctions in dyslexic children and adults. NeuroImage. 2011;56:1735–1742. doi: 10.1016/j.neuroimage.2011.02.040. [DOI] [PubMed] [Google Scholar]
- Rimol LM, Agartz I, Djurovic S, Brown AA, Roddey JC, Kohler AK, Mattingsdal M, Athanasiu L, Joyner AH, Schork NJ, Halgren E, Sundet K, Melle I, Dale AM, Andreassen OA. Sex-dependent association of common variants of microcephaly genes with brain structure. Proc. Natl. Acad. Sci. U. S. A. 2010;107:384–388. doi: 10.1073/pnas.0908454107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas HD, Liu AK, Hersch S, Glessner M, Ferrante RJ, Salat DH, van der Kouwe A, Jenkins BG, Dale AM, Fischl B. Regional and progressive thinning of the cortical ribbon in Huntington's disease. Neurology. 2002;58:695–701. doi: 10.1212/wnl.58.5.695. [DOI] [PubMed] [Google Scholar]
- Rosen GD, Bai J, Wang Y, Fiondella CG, Threlkeld SW, LoTurco JJ, Galaburda AM. Disruption of neuronal migration by RNAi of Dyx1c1 results in neocortical and hippocampal malformations. Cereb. Cortex. 2007;17(11):2562–2572. doi: 10.1093/cercor/bhl162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. NeuroImage. 2010;52:1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Rubinov M, Sporns O. Weight-conserving characterization of complex functional brain networks. NeuroImage. 2011;56:2068–2079. doi: 10.1016/j.neuroimage.2011.03.069. [DOI] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, Morris JC, Dale AM, Fischl B. Thinning of the cerebral cortex in aging. Cereb. Cortex. 2004;14:721–730. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- Sanabria-Diaz G, Melie-Garcia L, Iturria-Medina Y, Aleman-Gomez Y, Hernandez-Gonzalez G, Valdes-Urrutia L, Galan L, Valdes-Sosa P. Surface area and cortical thickness descriptors reveal different attributes of the structural human brain networks. NeuroImage. 2010;50:1497–1510. doi: 10.1016/j.neuroimage.2010.01.028. [DOI] [PubMed] [Google Scholar]
- Segonne F, Dale AM, Busa E, Glessner M, Salat D. A hybrid approach to the skull stripping problem in MRI. NeuroImage. 2004;22(3):1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Segonne F, Pacheco J, Fischl B. Geometrically accurate topology-correction of cortical surfaces using nonseparating loops. IEEE Trans. Med. Imaging. 2007;26:518–529. doi: 10.1109/TMI.2006.887364. [DOI] [PubMed] [Google Scholar]
- Seo SW, Im K, Lee JM, Kim YH, Kim ST, Kim SY, Yang DW, Kim SI, Cho YS, Na DL. Cortical thickness in single-versus multiple-domain amnestic mild cognitive impairment. NeuroImage. 2007;36:289–297. doi: 10.1016/j.neuroimage.2007.02.042. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE. Dyslexia. N. Engl. J. Med. 1998;338:307–312. doi: 10.1056/NEJM199801293380507. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Shaywitz BA. Dyslexia (specific reading disability) Pediatr. Rev. 2003;24:147–153. doi: 10.1542/pir.24-5-147. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Shaywitz BA, Fulbright RK, Skudlarski P, Mencl WE, Constable RT, Pugh KR, Holahan JM, Marchione KE, Fletcher JM, Lyon GR, Gore JC. Neural systems for compensation and persistence: young adult outcome of childhood reading disability. Biol. Psychiatry. 2003;54:25–33. doi: 10.1016/s0006-3223(02)01836-x. [DOI] [PubMed] [Google Scholar]
- Shaywitz BA, Shaywitz SE, Blachman BA, Pugh KR, Fulbright RK, Skudlarski P, Mencl WE, Constable RT, Holahan JM, Marchione KE, Fletcher JM, Lyon GR, Gore JC. Development of left occipitotemporal systems for skilled reading in children after a phonologically-based intervention. Biol. Psychiatry. 2004;55:926–933. doi: 10.1016/j.biopsych.2003.12.019. [DOI] [PubMed] [Google Scholar]
- Shibayama A, Cook EH, Feng J, Glanzmann C, Yan J, Craddock N, Jones IR, Goldman D, Heston LL, Sommer SS. MECP2 structural and 3′-UTR variants in schizophrenia, autism and other psychiatric diseases: a possible association with autism. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2004;128B:50–53. doi: 10.1002/ajmg.b.30016. [DOI] [PubMed] [Google Scholar]
- Simard D, Nadeau L. Fastest learning in small-world neural networks. Phys. Lett. A. 2005;336:8–15. [Google Scholar]
- Simos PG, Rezaie R, Fletcher JM, Juranek J, Papanicolaou AC. Neural correlates of sentence reading in children with reading difficulties. Neuroreport. 2011a;22:674–678. doi: 10.1097/WNR.0b013e328349ecf7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simos PG, Rezaie R, Fletcher JM, Juranek J, Passaro AD, Li Z, Cirino PT, Papanicolaou AC. Functional disruption of the brain mechanism for reading: effects of comorbidity and task difficulty among children with developmental learning problems. Neuropsychology. 2011b;25:520–534. doi: 10.1037/a0022550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans. Med. Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Specht K, Hugdahl K, Ofte S, Nygard M, Bjornerud A, Plante E, Helland T. Brain activation on pre-reading tasks reveals at-risk status for dyslexia in 6-year-old children. Scand. J. Psychol. 2009;50(1):79–91. doi: 10.1111/j.1467-9450.2008.00688.x. [DOI] [PubMed] [Google Scholar]
- Steinbrink C, Vogt K, Kastrup A, Muller HP, Juengling ED, Kassubek J, Riecker A. The contribution of white and gray matter differences to developmental dyslexia: insights from DTI and VBM at 3.0 T. Neuropsychologia. 2008;46:3170–3178. doi: 10.1016/j.neuropsychologia.2008.07.015. [DOI] [PubMed] [Google Scholar]
- Stoitsis J, Giannakakis GA, Papageorgiou C, Nikita KS, Rabavilas A, Anagnostopoulos D. Evidence of a posterior cingulate involvement (Brodmann area 31) in dyslexia: a study based on source localization algorithm of event-related potentials. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2008;32:733–738. doi: 10.1016/j.pnpbp.2007.11.022. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Black JM, Hulme C, Stanley LM, Kesler SR, Whitfield-Gabrieli S, Reiss A, Gabrieli JD, Hoeft F. The brain basis of the phonological deficit in dyslexia is independent of iq. Psychol. Sci. 2011;22:1442–1451. doi: 10.1177/0956797611419521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkeltaub PE, Gareau L, Flowers DL, Zeffiro TA, Eden GE. Development of neural mechanisms for reading. Nat. Neurosci. 2003;6(6):767–773. doi: 10.1038/nn1065. [DOI] [PubMed] [Google Scholar]
- Torppa M, Eklund K, van Bergen E, Lyytinen H. Parental literacy predicts children's literacy: a longitudinal family-risk study. Dyslexia. 2011;17(4):339–355. doi: 10.1002/dys.437. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. NeuroImage. 2002;16:765–780. doi: 10.1006/nimg.2002.1131. [DOI] [PubMed] [Google Scholar]
- Uchino J, Suzuki M, Hoshino K, Nomura Y, Segawa M. Development of language in Rett syndrome. Brain Dev. 2001;23(S1):S233–S235. doi: 10.1016/s0387-7604(01)00367-9. [DOI] [PubMed] [Google Scholar]
- van Bergen E, de Jong PF, Plakas A, Maassen B, van der Leij A. Child and parental literacy levels within families with a history of dyslexia. J. Child Psychol. Psychiatry. 2012;53(1):28–36. doi: 10.1111/j.1469-7610.2011.02418.x. [DOI] [PubMed] [Google Scholar]
- van den Heuvel MP, Stam CJ, Kahn RS, Hulshoff Pol HE. Efficiency of functional brain networks and intellectual performance. J. Neurosci. 2009;29:7619–7624. doi: 10.1523/JNEUROSCI.1443-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Mark S, Bucher K, Maurer U, Schulz E, Brem S, Buckelmuller J, Kronbichler M, Loenneker T, Klaver P, Martin E, Brandeis D. Children with dyslexia lack multiple specializations along the visual word-form (VWF) system. NeuroImage. 2009;47:1940–1949. doi: 10.1016/j.neuroimage.2009.05.021. [DOI] [PubMed] [Google Scholar]
- van der Mark S, Klaver P, Bucher K, Maurer U, Schulz E, Brem S, Martin E, Brandeis D. The left occipitotemporal system in reading: disruption of focal fMRI connectivity to left inferior frontal and inferior parietal language areas in children with dyslexia. NeuroImage. 2011;54:2426–2436. doi: 10.1016/j.neuroimage.2010.10.002. [DOI] [PubMed] [Google Scholar]
- van Wijk BC, Stam CJ, Daffertshofer A. Comparing brain networks of different size and connectivity density using graph theory. PLoS One. 2010;5:e13701. doi: 10.1371/journal.pone.0013701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannest JJ, Karunanayaka PR, Altaye M, Schmithorst VJ, Plante EM, Eaton KJ, Rasmussen JM, Holland SK. Comparison of fMRI data from passive listening and active-response story processing tasks in children. J. Magn. Reson. Imaging. 2009;29:971–976. doi: 10.1002/jmri.21694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vawter MP, Harvey PD, DeLisi LE. Dysregulation of X-linked gene expression in Klinefelter's syndrome and association with verbal cognition. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2007;144B:728–734. doi: 10.1002/ajmg.b.30454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicari S, Finzi A, Menghini D, Marotta L, Petrosini L. Do children with developmental dyslexia have an implicit learning deficit? J. Neurol. Neurosurg. Psychiatry. 2005;76:1392–1397. doi: 10.1136/jnnp.2004.061093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigneau M, Jobard G, Mazoyer B. Word and non-word reading: what role for the visual word form area? NeuroImage. 2005;27:694–705. doi: 10.1016/j.neuroimage.2005.04.038. [DOI] [PubMed] [Google Scholar]
- Vincent A, Deacon R, Dalton P, Salmond C, Blamire AM, Pendlebury S, Johansen-Berg H, et al. Maternal antibody-mediated dyslexia? Evidence for a pathogenic serum factor in a mother of two dyslexic children shown by transfer to mice using behavioural studies and magnetic resonance spectroscopy. J. Neuroimmunol. 2002;130(1–2):243–247. doi: 10.1016/s0165-5728(02)00226-6. [DOI] [PubMed] [Google Scholar]
- Wadsworth SJ, Corley RP, Hewitt JK, Plomin R, DeFries JC. Parent–offspring resemblance for reading performance at 7, 12 and 16 years of age in the Colorado Adoption Project. J. Child Psychol. Psychiatry. 2002;43(6):769–774. doi: 10.1111/1469-7610.00085. [DOI] [PubMed] [Google Scholar]
- Wang L, Goldstein FC, Veledar E, Levey AL, Lah JJ, Meltzer CC, Holder CA, Mao H. Alterations in cortical thickness and white matter integrity in mild cognitive impairment measured by whole-brain cortical thickness mapping and diffusion tensor. Am. J. Neuroradiol. 2009;30:893–899. doi: 10.3174/ajnr.A1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts D, Strogatz S. Collective dynamics of small-world networks. Nature. 1998;393:440–442. doi: 10.1038/30918. [DOI] [PubMed] [Google Scholar]
- Whitney C, Huber W, Klann J, Weis S, Krach S, Kircher T. Neural correlates of narrative shifts during auditory story comprehension. NeuroImage. 2009;47:360–366. doi: 10.1016/j.neuroimage.2009.04.037. [DOI] [PubMed] [Google Scholar]
- Wright IC, Sharma T, Ellison ZR, McGuire PK, Friston KJ, Brammer MJ, Murray RM, Bullmore ET. Supra-regional brain systems and the neuropathology of schizophrenia. Cereb. Cortex. 1999;9:366–378. doi: 10.1093/cercor/9.4.366. [DOI] [PubMed] [Google Scholar]
- Zhang K, Sejnowsky TJ. A universal scaling law between gray matter and white matter of cerebral cortex. Proc. Natl. Acad. Sci. U. S. A. 2000;97:5621–5626. doi: 10.1073/pnas.090504197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler JC, Goswami U. Reading acquisition, developmental dyslexia, and skilled reading across languages: a psycholinguistic grain size theory. Psychol. Bull. 2005;131:3–29. doi: 10.1037/0033-2909.131.1.3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.