Abstract
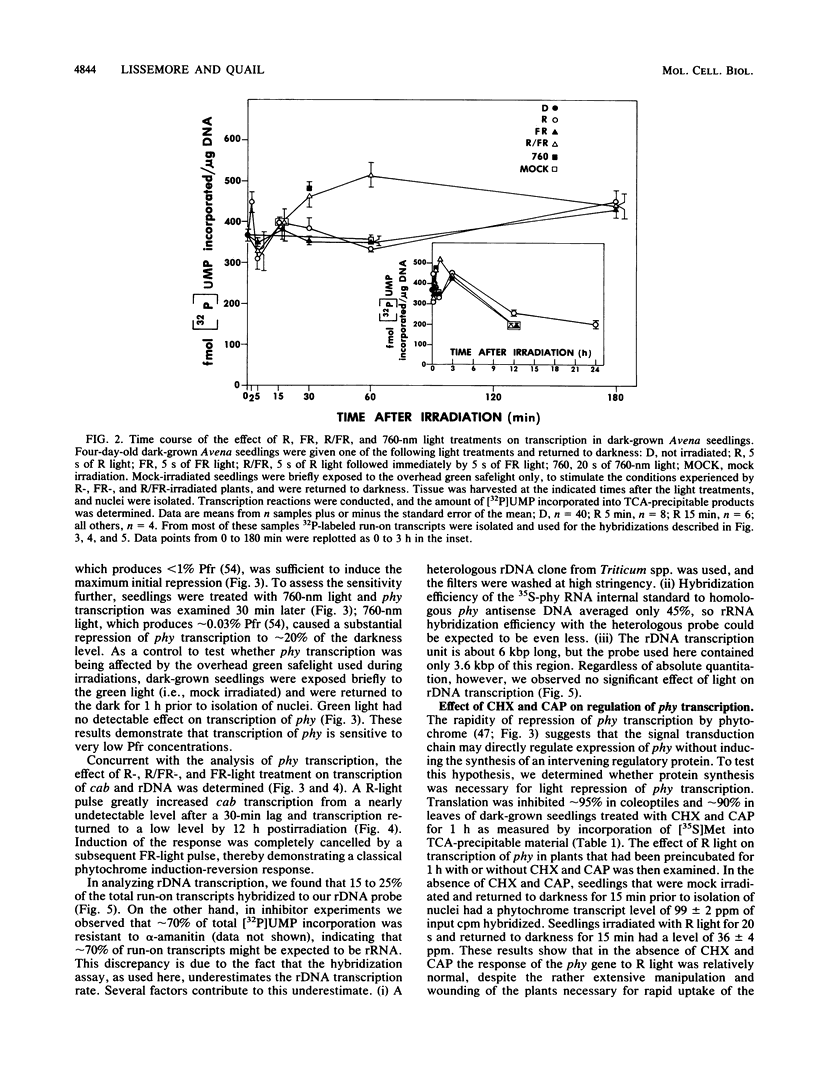
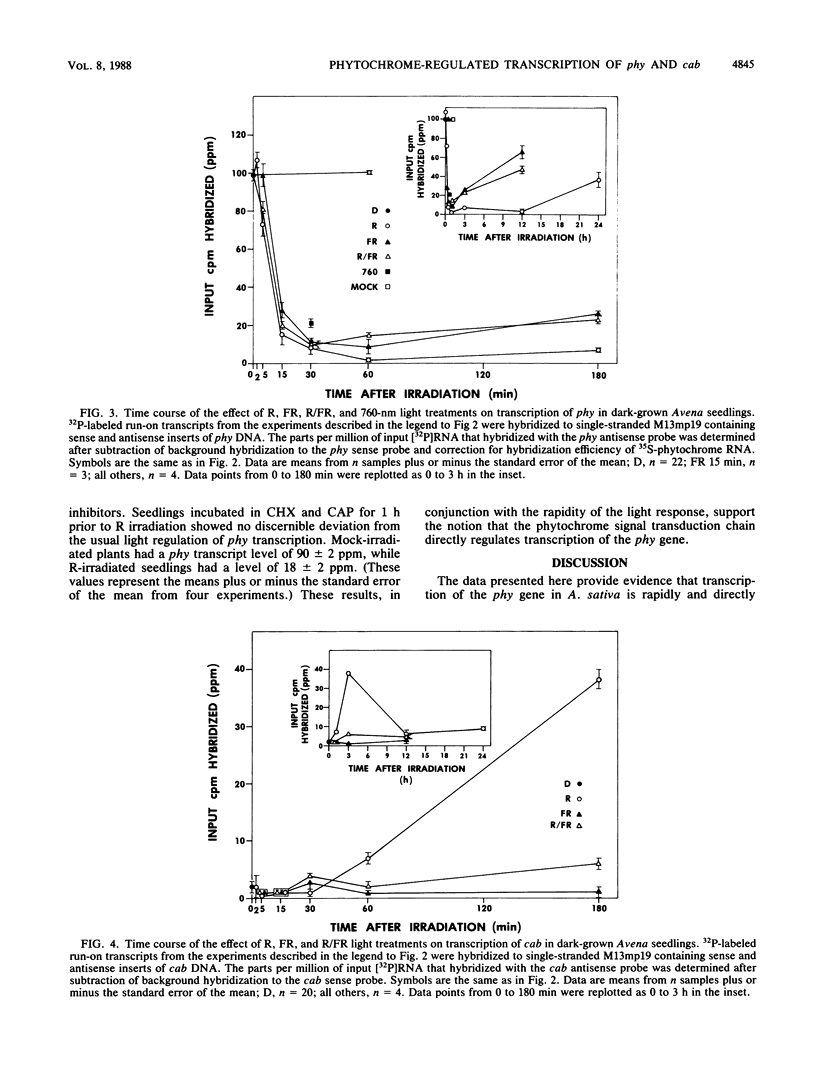
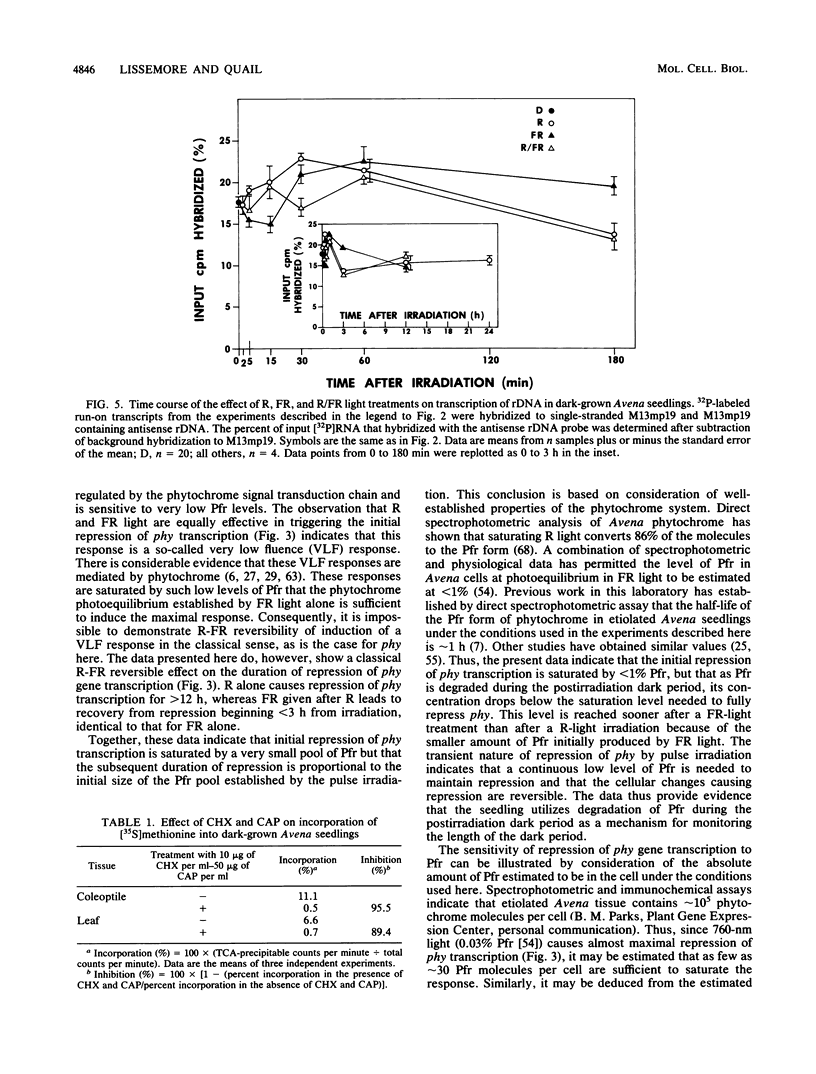
We have examined phytochrome-regulated transcription of phytochrome (phy) and chlorophyll a/b binding protein (cab) genes in dark-grown Avena seedlings by using run-on transcription in isolated nuclei. Kinetic analysis of phy transcription following pulse-light treatments to produce various amounts of Pfr, the active form of phytochrome, leads to these conclusions. (i) Transcription decreases rapidly (discernible within 5 min) after Pfr formation, reaching an essentially undetectable level by 1 h. (ii) The response is very sensitive; less than 1% Pfr is sufficient to produce maximum feedback repression over the first 30 min. (iii) The duration of transcriptional repression is proportional to the Pfr concentration; derepression begins once the concentration falls below some saturation level because of degradation of Pfr. Concurrent analysis of cab transcription leads to these conclusions. (i) After Pfr formation, transcription increases approximately 10-fold by 3 h, but this response is not detectable until after a 30-min lag. (ii) Detectable induction of cab requires a greater than 30-fold-higher Pfr level than is needed to repress phy expression. (iii) Transcription returns to the preirradiation level considerably sooner than does phy transcription (less than 12 h versus greater than 24 h respectively), indicating that a high level of Pfr is needed to sustain the increased transcription of cab. Taken together, these results suggest that differences in the phytochrome signal transduction pathway are responsible for the distinct patterns of regulation of these genes. Full repression of phy occurs even when protein synthesis is inhibited greater than 90% by cycloheximide and chloramphenicol. In conjunction with the rapidity of the response to Pfr, this result provides evidence that feedback repression of phy gene transcription does not require expression of an intervening regulatory gene(s). Thus, phy is the first gene for which there is evidence for direct control of transcription by the phytochrome signal transduction chain.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashburner M., Bonner J. J. The induction of gene activity in drosophilia by heat shock. Cell. 1979 Jun;17(2):241–254. doi: 10.1016/0092-8674(79)90150-8. [DOI] [PubMed] [Google Scholar]
- Beach L. R., Spencer D., Randall P. J., Higgins T. J. Transcriptional and post-transcriptional regulation of storage protein gene expression in sulfur-deficient pea seeds. Nucleic Acids Res. 1985 Feb 11;13(3):999–1013. doi: 10.1093/nar/13.3.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry-Lowe S. L., Meagher R. B. Transcriptional regulation of a gene encoding the small subunit of ribulose-1,5-bisphosphate carboxylase in soybean tissue is linked to the phytochrome response. Mol Cell Biol. 1985 Aug;5(8):1910–1917. doi: 10.1128/mcb.5.8.1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbert J. T., Hershey H. P., Quail P. H. Autoregulatory control of translatable phytochrome mRNA levels. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2248–2252. doi: 10.1073/pnas.80.8.2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst D., Oesterhelt D. Purified phytochrome influences in vitro transcription in rye nuclei. EMBO J. 1984 Dec 20;3(13):3075–3078. doi: 10.1002/j.1460-2075.1984.tb02261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. M., Hollenberg S. M. Zinc fingers: gilt by association. Cell. 1988 Jan 15;52(1):1–3. doi: 10.1016/0092-8674(88)90522-3. [DOI] [PubMed] [Google Scholar]
- Fluhr R., Chua N. H. Developmental regulation of two genes encoding ribulose-bisphosphate carboxylase small subunit in pea and transgenic petunia plants: Phytochrome response and blue-light induction. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2358–2362. doi: 10.1073/pnas.83.8.2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluhr R., Kuhlemeier C., Nagy F., Chua N. H. Organ-specific and light-induced expression of plant genes. Science. 1986 May 30;232(4754):1106–1112. doi: 10.1126/science.232.4754.1106. [DOI] [PubMed] [Google Scholar]
- Friedman R. L., Manly S. P., McMahon M., Kerr I. M., Stark G. R. Transcriptional and posttranscriptional regulation of interferon-induced gene expression in human cells. Cell. 1984 Oct;38(3):745–755. doi: 10.1016/0092-8674(84)90270-8. [DOI] [PubMed] [Google Scholar]
- Gallagher T. F., Ellis R. J. Light-stimulated transcription of genes for two chloroplast polypeptides in isolated pea leaf nuclei. EMBO J. 1982;1(12):1493–1498. doi: 10.1002/j.1460-2075.1982.tb01345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach W. L., Bedbrook J. R. Cloning and characterization of ribosomal RNA genes from wheat and barley. Nucleic Acids Res. 1979 Dec 11;7(7):1869–1885. doi: 10.1093/nar/7.7.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B., Greene L. A. Stimulation of neuronal acetylcholine receptors induces rapid gene transcription. Science. 1986 Oct 3;234(4772):80–83. doi: 10.1126/science.3749894. [DOI] [PubMed] [Google Scholar]
- Hagen G., Guilfoyle T. J. Rapid induction of selective transcription by auxins. Mol Cell Biol. 1985 Jun;5(6):1197–1203. doi: 10.1128/mcb.5.6.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershey H. P., Barker R. F., Idler K. B., Lissemore J. L., Quail P. H. Analysis of cloned cDNA and genomic sequences for phytochrome: complete amino acid sequences for two gene products expressed in etiolated Avena. Nucleic Acids Res. 1985 Dec 9;13(23):8543–8559. doi: 10.1093/nar/13.23.8543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt R. E., Pratt L. H. Phytochrome radioimmunoassay. Plant Physiol. 1979 Aug;64(2):327–331. doi: 10.1104/pp.64.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman L. S., Briggs W. R., Thompson W. F. Phytochrome control of specific mRNA levels in developing pea buds : the presence of both very low fluence and low fluence responses. Plant Physiol. 1985 Jun;78(2):388–393. doi: 10.1104/pp.78.2.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Lawton M. A., Lamb C. J. Transcriptional activation of plant defense genes by fungal elicitor, wounding, and infection. Mol Cell Biol. 1987 Jan;7(1):335–341. doi: 10.1128/mcb.7.1.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Mösinger E., Batschauer A., Apel K., Schäfer E., Briggs W. R. Phytochrome regulation of greening in barley : effects on mRNA abundance and on transcriptional activity of isolated nuclei. Plant Physiol. 1988 Mar;86(3):706–710. doi: 10.1104/pp.86.3.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mösinger E., Batschauer A., Schäfer E., Apel K. Phytochrome control of in vitro transcription of specific genes in isolated nuclei from barley (Hordeum vulgare). Eur J Biochem. 1985 Feb 15;147(1):137–142. doi: 10.1111/j.1432-1033.1985.tb08729.x. [DOI] [PubMed] [Google Scholar]
- Nagy F., Boutry M., Hsu M. Y., Wong M., Chua N. H. The 5'-proximal region of the wheat Cab-1 gene contains a 268-bp enhancer-like sequence for phytochrome response. EMBO J. 1987 Sep;6(9):2537–2542. doi: 10.1002/j.1460-2075.1987.tb02541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy F., Kay S. A., Boutry M., Hsu M. Y., Chua N. H. Phytochrome-controlled expression of a wheat Cab gene in transgenic tobacco seedlings. EMBO J. 1986 Jun;5(6):1119–1124. doi: 10.1002/j.1460-2075.1986.tb04335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Quail P. H., Colbert J. T., Peters N. K., Christensen A. H., Sharrock R. A., Lissemore J. L. Phytochrome and the regulation of the expression of its genes. Philos Trans R Soc Lond B Biol Sci. 1986 Nov 17;314(1166):469–480. doi: 10.1098/rstb.1986.0066. [DOI] [PubMed] [Google Scholar]
- Quail P. H., Schäfer E., Marmé D. De novo synthesis of phytochrome in pumpkin hooks. Plant Physiol. 1973 Aug;52(2):124–127. doi: 10.1104/pp.52.2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharrock R. A., Lissemore J. L., Quail P. H. Nucleotide and amino acid sequence of a Cucurbita phytochrome cDNA clone: identification of conserved features by comparison with Avena phytochrome. Gene. 1986;47(2-3):287–295. doi: 10.1016/0378-1119(86)90072-7. [DOI] [PubMed] [Google Scholar]
- Shirley B. W., Berry-Lowe S. L., Rogers S. G., Flick J. S., Horsch R., Fraley R. T., Meagher R. B. 5' proximal sequences of a soybean ribulose-1,5-bisphosphate carboxylase small subunit gene direct light and phytochrome controlled transcription. Nucleic Acids Res. 1987 Aug 25;15(16):6501–6514. doi: 10.1093/nar/15.16.6501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverthorne J., Tobin E. M. Demonstration of transcriptional regulation of specific genes by phytochrome action. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1112–1116. doi: 10.1073/pnas.81.4.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somssich I. E., Schmelzer E., Bollmann J., Hahlbrock K. Rapid activation by fungal elicitor of genes encoding "pathogenesis-related" proteins in cultured parsley cells. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2427–2430. doi: 10.1073/pnas.83.8.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theologis A., Huynh T. V., Davis R. W. Rapid induction of specific mRNAs by auxin in pea epicotyl tissue. J Mol Biol. 1985 May 5;183(1):53–68. doi: 10.1016/0022-2836(85)90280-3. [DOI] [PubMed] [Google Scholar]
- Ucker D. S., Yamamoto K. R. Early events in the stimulation of mammary tumor virus RNA synthesis by glucocorticoids. Novel assays of transcription rates. J Biol Chem. 1984 Jun 25;259(12):7416–7420. [PubMed] [Google Scholar]
- Vierstra R. D., Quail P. H. Photochemistry of 124 kilodalton Avena phytochrome in vitro. Plant Physiol. 1983 May;72(1):264–267. doi: 10.1104/pp.72.1.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong Y. S., Cheng H. C., Walsh D. A., Lagarias J. C. Phosphorylation of Avena phytochrome in vitro as a probe of light-induced conformational changes. J Biol Chem. 1986 Sep 15;261(26):12089–12097. [PubMed] [Google Scholar]
- Yamamoto K. R. Steroid receptor regulated transcription of specific genes and gene networks. Annu Rev Genet. 1985;19:209–252. doi: 10.1146/annurev.ge.19.120185.001233. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]



