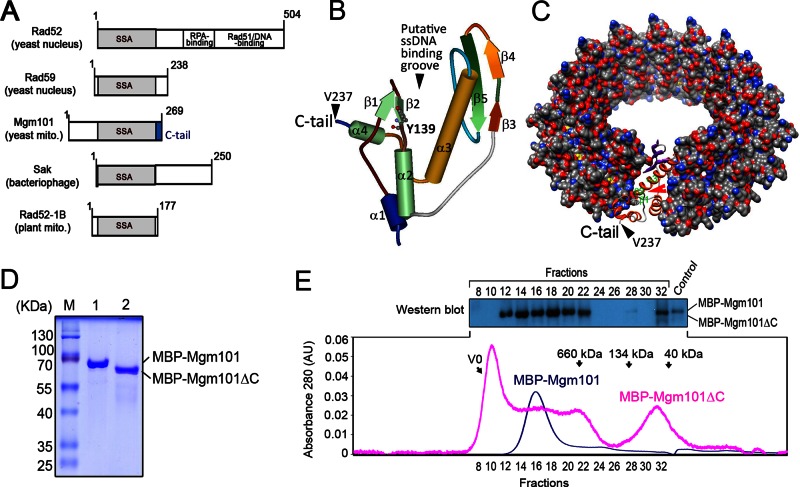
FIGURE 1:
The C-tail deletion destabilizes the oligomeric state of Mgm101. (A) Domain organization of SSAPs from various species. All SSAPs contain the conserved SSA domain (shown in gray). The Mgm101-specific C-tail is shown in blue. (B) Solid ribbon structure of Mgm101115-237 modeled on the N-terminal ssDNA-binding domain of human Rad52 (1H2I). The projected position of Tyr-139 is represented by scaled balls and sticks. (C) Surface representation model of an Mgm101 14-mer ring modeled on the crystal structure of the human Rad52 (1H2I). The red arrow indicates the putative ssDNA-binding groove predicted for Rad52. The black arrow indicates that Val237 is displayed on the surface of the ring, which is followed by the C-tail. (D) SDS–PAGE showing purified Mbp-Mgm101ΔC after amylose resin affinity chromatography (lane 2) compared with a wild-type fusion control (lane 1). (E) Size exclusion chromatography of MBP-Mgm101ΔC (pink) with corresponding fractions analyzed by Western blot as depicted. Wild-type fusion control (blue) is a monodisperse peak of ∼940 kDa, in contrast to the polydisperse MBP-Mgm101ΔC. Note that no signal is detected by Western blot for MBP-Mgm101ΔC present in the void volume, likely due to precipitation of the protein aggregates in the solution.