FIGURE 3:
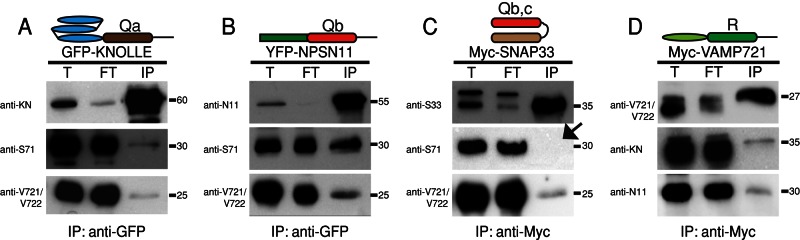
Interaction analysis of candidate SNARE proteins involved in cytokinesis. GFP-KNOLLE (A), YFP-NPSN11 (B), Myc-SNAP33 (C), and Myc-VAMP721 (D) were immunoprecipitated from total protein extracts of Arabidopsis inflorescences. (A) GFP-KNOLLE coprecipitated SYP71 and R-SNAREs VAMP721 and VAMP722. (B) YFP-NPSN11 coprecipitated SYP71 and R-SNAREs VAMP721 and VAMP722. (C) Myc-SNAP33 coprecipitated R-SNAREs VAMP721 and VAMP722 but not SYP71 (arrow). The upper band in (T) and (FT) represents a protein that cross-reacted with the SNAP33 antiserum and was not precipitated. (D) Myc-VAMP721 coprecipitated KNOLLE and NPSN11. Protein was detected in total extract (T), flowthrough (FT), and immunoprecipitate (IP) by Western blot using anti-SNAP33 (S33), anti-NPSN11 (N11), anti-KNOLLE (KN), anti-SYP71 (S71), or anti-VAMP721/722 (V721/722) antiserum. Note that there is no interaction of endogenous KNOLLE, NPSN11, SNAP33, or VAMP721/722 with their GFP-, YFP-, or Myc-tagged fusion protein; the immunoblots show only GFP-KNOLLE, YFP-NPSN11, Myc-SNAP33, and Myc-VAMP721/722, as indicated by the molecular weight (in kilodaltons) on the right side.
