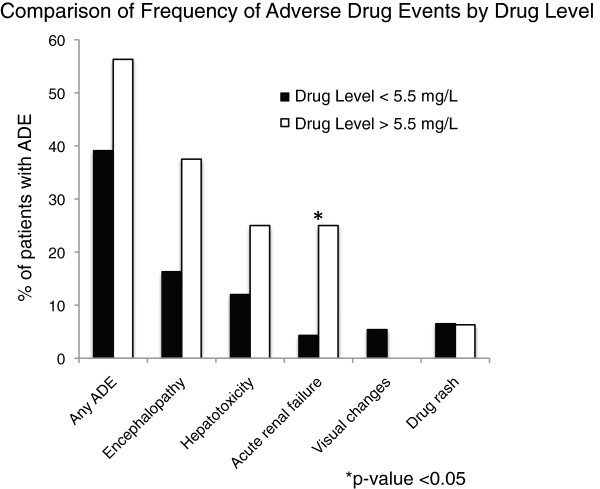
Figure 4.
Comparison of frequency of adverse drug events in patients with and without supratherapeutic drug levels, defined as a level > 5.5 mg/L. The y-axis represents the percentage of patients with adverse drug events. The total number of adverse drug events, as well as individual drug events, are noted on the x-axis. There was no statistically significant difference in the frequency of overall adverse drug events, or of encephalopathy, hepatoxicity, skin rash, or visual changes among patients with and without supratherapeutic voriconazole drug levels. There was a statistically significant difference in incidence of acute renal failure (*) between the two groups.