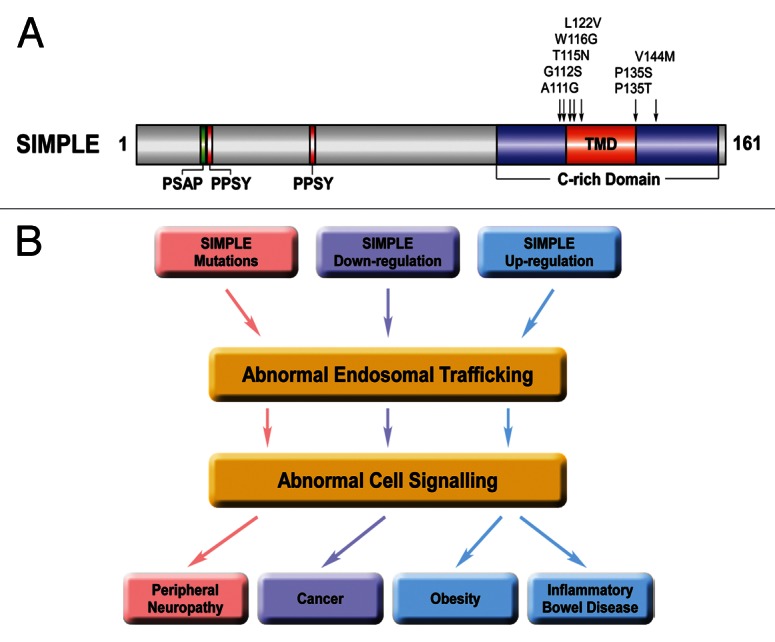
Figure 1. Endosomal trafficking and signaling dysregulation as a potential pathogenic mechanism in CMT and other diseases that involve SIMPLE dysfunction. (A) Domain structure of SIMPLE and mutations found in CMT1C patients. PSAP, predicted TSG101-binding site; PPSY, predicted NEDD4-binding site; C-rich domain, cysteine-rich domain; TMD, predicted transmembrane domain. The locations of CMT1C-linked SIMPLE mutations are indicated on the domain structure. (B) Potential pathogenic roles of SIMPLE dysfunction in CMT, cancer, obesity and inflammatory bowel diseases. Our recent work24 suggests a pathogenic pathway by which CMT1C-linked SIMPLE mutations2-6 cause demyelinating peripheral neuropathy by disrupting endosome-to-lysosome trafficking and signaling attenuation of NRG1-activated ErbB2/ErbB3 receptors and consequently prolonging their signaling to downstream pathways in Schwann cells (colored in pink). Downregulation of SIMPLE expression found in several types of cancer11-14 may contribute to the process of malignant transformation by impairing endosome-to-lysosome trafficking and signaling attenuation of mitogenic signaling receptors (colored in lavender). Upregulation of SIMPLE expression found in obesity15 and inflammatory bowel diseases16 may contribute to the pathogenesis or progression of these diseases by altering endosomal trafficking and intracellular signaling (colored in blue).
