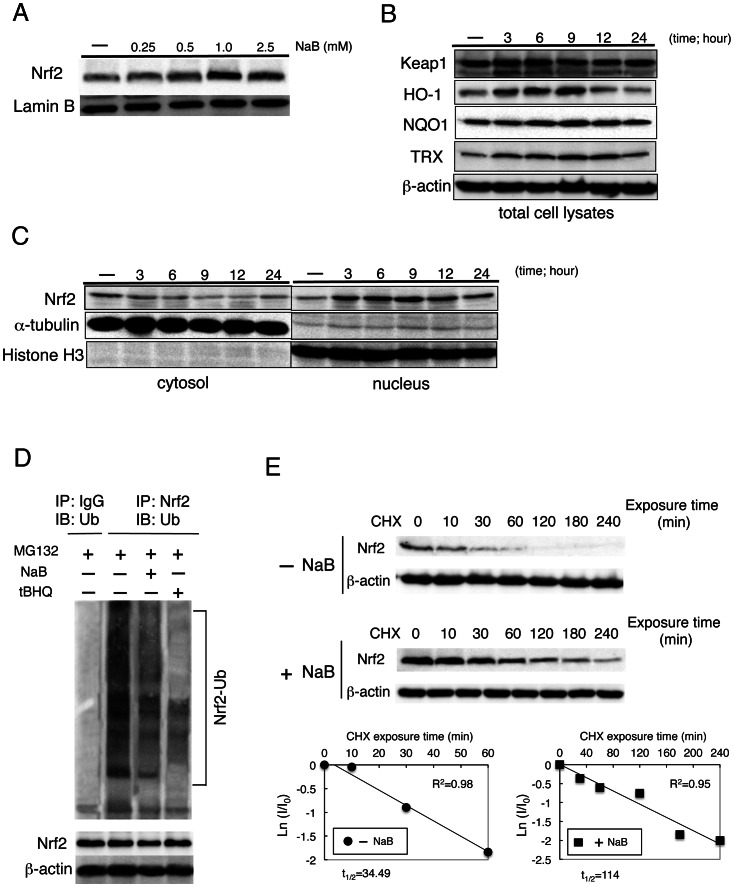
Figure 5. Sodium butyrate (NaB) induced Nrf2 expression and nuclear accumulation.
(A) Dose-dependent effects of NaB on the expression of Nrf2 protein in HepG2 cells maintained in serum-free medium for 24 h and then treated with NaB at described concentrations for 6 h. Lamin B expression was used as a loading control. (B) Expression of Keap1, HO-1, NQO1, and TRX was examined by western blot analysis at 6 h. HepG2 cells were treated with 1.5 mM NaB at the indicated time points. (C) Nuclear accumulation of Nrf2 was stimulated by 1.5-mM NaB treatment of cells for the indicated time. Cytoplasmic and nucleic extracts were prepared and subjected to western blot analysis. Anti-α-tubulin and anti-histone H3 antibodies were used as markers for the cytoplasmic and nuclear extracts, respectively. (D) Ubiquitination of endogenous Nrf2 was assessed in HepG2 cells treated with DMSO, 1.5 mM NaB or 100 µM tBHQ for 9 h, along with 10 µM MG132. Nrf2 was immunoprecipitated with an anti-Nrf2 antibody and ubiquitinated Nrf2 was detected with an anti-ubiquitin antibody. (E) Post-transcriptional regulation of both the steady-state level and half-life of Nrf2 protein was evaluated. CHX (100 µM) was added to block protein synthesis. Cells were lysed at the indicated time points, and cell lysates were subjected to western blot analysis with anti-Nrf2 and anti-β-actin antibodies (upper panels). Lower panels depict the natural logarithm of the relative levels of the Nrf2 protein as a function of CHX chase time in the absence or presence of 1.5 mM NaB. The protein half-life has been determined in the linear range of the degradation curve.