Figure 1. Identification of PDZ2 of SAP97 as a binding partner to the C-tail of the ß1-AR.
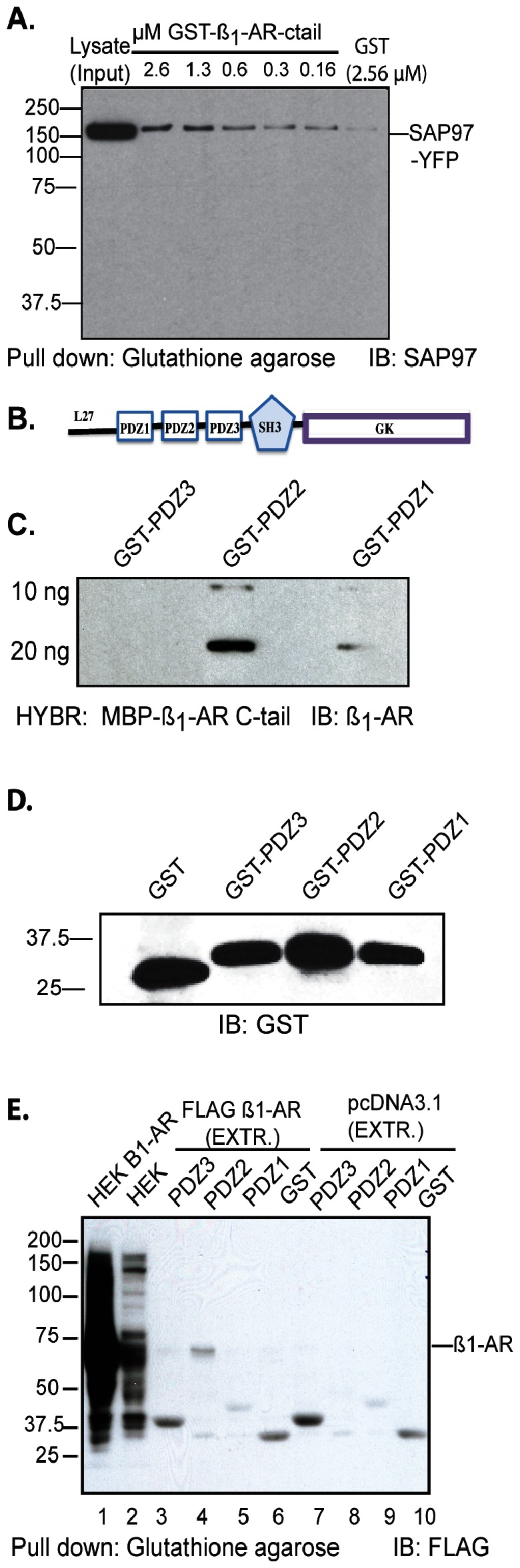
A, increasing concentrations of GST-tagged ß1-ARC-tail or GST were screened for interactions with SAP97 by GST pulldown using lysates from KEK-293 cell expressing SAP97-YFP. GST pull-downs (IP) were immunoblotted (IB) for SAP97 using a SAP97 antibody from Enzo Life Sciences. The optical density of each band was plotted against µM of ß1-AR protein to estimate the concentration of ß1-AR protein at 50% of the maximal optical density. These values from n = 3 experiments were used to calculate the apparent EC50 of their interaction. Input represents ∼4% of the lysate. B, schematic diagram of the protein-protein interacting domains of SAP97. C, 10 µl or 20 µl of a 1 µg/ml stock solution of GST-tagged PDZ1, PDZ2 or PDZ3 of SAP97 were slot-blotted onto nitrocellulose membranes. The membranes were hybridized with 16 µg/ml (0.34 µM) of MBP-ß1-AR(425–477) fusion protein. The binding of the ß1-AR to each PDZ was detected with the sc-567 anti-ß1-AR antibody (Santa Cruz). D, Western blot (IB) of purified GST or GST-PDZ1, -PDZ2 or -PDZ3 fusions. E, Equal amounts of protein lysates from HEK-293 cells expressing either the empty pcDNA 3.1 or FLAG WT ß1-AR were mixed with 10 µg of GST or 12.5 µg of the indicated GST-tagged PDZ in a total volume of 1 ml (0.38 µM of GST or GST-PDZ). Then ∼4% input lysates (lanes 1, 2) or GST pull-downs (IP) from cells that expressed the WT ß1-AR (lanes 3–6) or the empty vector (lanes 7–10) were subjected to Western blotting (IB) and probed with the anti-FLAG antibody.
