Abstract
This case report documents the use of the CIMAvax Epidermal Growth Factor vaccine regimen in a 54 y old female with stage IIIb non-small cell lung carcinoma. Even after 48 mo since diagnosis her ECOG performance remains at zero. Further, this report documents a reaction to the vaccine of grade 3 severity not previously documented.
Keywords: lung, cimavax, epidermal growth factor (EGF), non small cell lung cancer (NSCLC)
Introduction
Over 75% of patients with NSCLC present with stage III or IV disease.1 At these stages concurrent chemoradiotherapy or palliative chemotherapy are the preferred treatments.2 Used optimally, these conventional therapies offer a median survival time of approximately one year from the time of diagnosis.3
By comparison patients undergoing the experimental CIMAvax (Center for Genetic Engineering and Biotechnology) Epidermal Growth Factor (EGF) vaccine treatment regimen have demonstrated significant increase in survival time. It should be noted that the following quoted survival times in the CIMAvax trials are measured from time of inclusion and not time from diagnosis. Pooled data from three Phase I/II randomized trials (n = 83, all of whom had had at least one line of conventional treatment) showed that, on average, vaccinated patients had a mean survival time of almost double that of concurrent unvaccinated controls (9.13 vs. 4.85 mo, p < 0.02).4 A larger scale Phase II randomized controlled trial (n = 80) produced similar results.5 It added that vaccinated patients under the age of 60 had an especially improved median survival time over control subjects (11.57 vs. 5.33 mo, p < 0.02). Due to its remarkable results, the vaccine has received Sanitary Registration in Cuba for advanced NSCLC. Our case took part in a Phase III trial currently underway.
The CIMAvax vaccine regimen works by inducing the patient’s own immune system to produce antibodies against self-made EGF ligand. EGF is a mitogenic factor which plays an important role in cell proliferation through various pathways including KRas and JAK/STAT.6 In NSCLC its overexpression positively correlates to shortened survival time.7 By stimulating the immune system against circulating autologous EGF these tumorigenic pathways are disrupted.
Survival time has been found to be positively tied to the degree of seroconversion.4,5 Those whose antibody titers rise above 1:64 000 sera dilution are termed Super Good Antibody Responders (sGAR). By contrast those that seroconvert but do not reach 1:4000 sera dilution are termed Poor Antibody Responders (PAR).8 Those whose antibody titers are between PAR and sGAR are named Good Antibody Responders (GAR). sGAR patients survive longer than GAR. GAR patients in turn survive longer than PAR.8,9
An emerging discovery which may improve patient survival centers around the so-called “loop B” component of the EGF ligand. This peptide facilitates EGF binding to its receptor in the tumorigenic pathway. One trial found that patients in whom immune action centered against this peptide had a better prognosis.10,11
The CIMAvax regimen is still evolving. Issues such as scheduling, dosages and length of treatment are continually undergoing re-evaluation. Our case received cyclophosphamide pretreatment before her vaccination schedule (Table 1). 80% of those given cyclophosphamide before the CIMAvax course will demonstrate some anti-EGF activity.4 Effectiveness is further enhanced if the vaccine is injected over multiple sites as our case was.9 Doing so may involve more lymph node regions.12
Table 1. Madam FNM's CIMAvax regimen.
| Time | Action |
|---|---|
| Day minus 3 |
290mg cyclophosphamide |
| Day 0, 14, 28 |
200ug CIMAvax injected over 4 sites (ie 50ug per deltoid and gluteus) |
| Every 28 d until withdrawal from regimen | 200ug CIMAvax injected over 4 sites (ie 50ug per deltoid and gluteus) |
The vaccine itself (developed and produced in the Center of Molecular Immunology) contains human recombinant EGF and a carrier protein in an adjuvant. Both the EGF and carrier protein (P64K) are produced by the Center for Genetic Engineering and Biotechnology. Trials have shown that the addition of P64K and adjuvant—Montanide ISA 51 (Seppic)—increase seroconversion rates while being low in immunogenicity themselves.4,13–15
Documented side effects are mild. These include tremors, headache, constitutional symptoms and arthralgia.8,15 The theoretical side effect of autoimmunity has not been recorded in vivo.16
The objective of this article is to introduce practitioners to the potential of this vaccine, and describe a side effect not previously documented.
Case Presentation
54 y old Madam FNM presented in December 2007 with a 2 mo history of worsening hemoptysis. This was associated with loss of weight and appetite and bone pain. A CT chest scan showed a ‘large, lobulated, heterogeneously enhancing mass’ at the posterobasal segment of the left lower lobe of the lung measuring 9 cm by 5.8 cm by 7.2 cm. Another smaller spiculated lesion was noted in the left upper lobe. A biopsy confirmed an infiltrating, moderately differentiated squamous cell carcinoma. The CT scan was negative for chest wall muscle involvement. Her bone scan was negative. She was diagnosed with stage T4N1M0-IIIb NSCLC.
Madam FNM was treated with 3 cycles of paclitaxel (260 mg) and carboplatin (415 mg) over 3 mo. This reduced the tumor to 7 cm by 6 cm by 5 cm. Concurrent chemoradiotherapy, 2 cycles of cisplatin (50 mg) and 60 Gy of radiation over 30 fractions then followed.
Two months after her chemoradiotherapy had ceased, Madam FNM agreed to take part in the CIMAvax regimen (Table 1). A pre-regimen CT scan demonstrated the lower lobe lesion to be 3 cm by 3 cm (Fig. 1). The left upper lobe lesion was less than 1 cm diameter with a loculated pleural effusion secondary to radiation. After 3 mo the tumor had shrunk to 2 cm by 2.1 cm (Fig. 2). By 6 mo the tumor shrank 30% from its original volume to 1.5 cm by 2.3 cm before stabilizing. In the same period her pleural effusion diminished while local lymph nodes became smaller.
Figure 1.
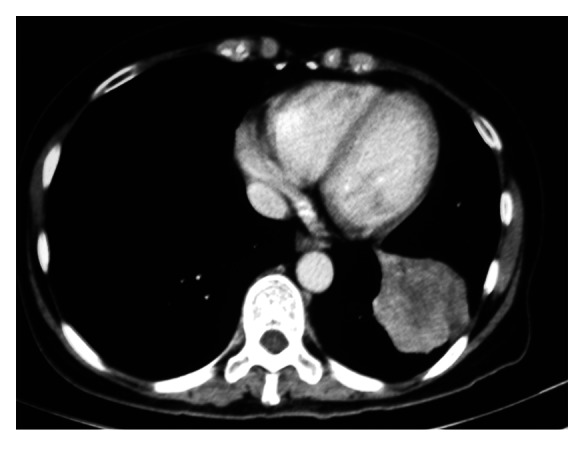
Pre-regimen CT chest, left lower lobe lesion 3 cm by 3 cm.
Figure 2.
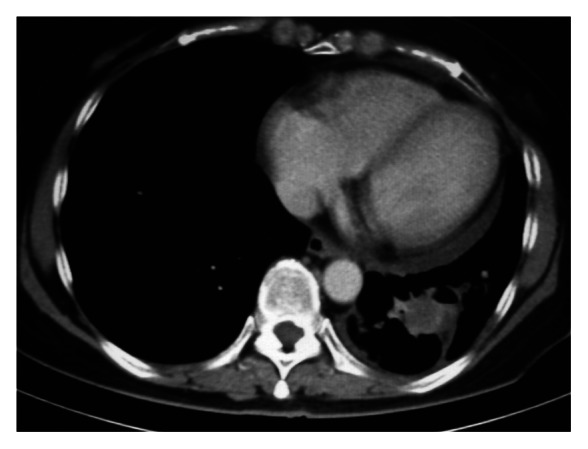
CT chest (month 3 from CIMAvax commencement), left lower lobe lesion 2 cm by 2.1 cm.
Madam FNM did not report any side effects during the course of the regimen until her 17th round of injections. Within minutes of her injections she experienced an “excruciating” pain localized to the lumbar and loin in a similar distribution to that of renal colic. This is classed as a vaccination-related Grade 3 reaction.17 It subsided after 10 min after treatment with IV chlorphenamine 10mg, IV hydrocortisone 200mg and IV tramadol 50mg. Because of this she decided to withdraw from the CIMAvax regime, and since not experienced any further episodes of pain. The patient was not rechallenged with CIMAvax. Allergy testing was not performed.
A CT chest scan was performed three months after cessation (month 18) of treatment. There were “no obvious changes” in the size of the tumor since her last scan 6 mo prior (Fig. 3). At last followup—28 mo after stopping the vaccine—Madam FNM was well and enjoying stable health. Her ECOG status had remained at 0. At this point she had survived for 48 mo since diagnosis.
Figure 3.
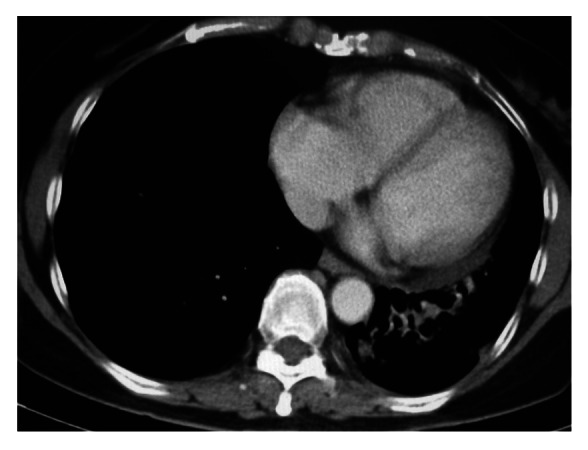
CT chest (month 18 from CIMAvax commencement), left lower lobe lesion 1.5 cm by 2.3 cm.
Discussion
Madam FNM’s survival—48 mo since diagnosis—has been remarkable. Ongoing vaccination is necessary to boost antibody titers back to peak levels.4 Therefore it is interesting that her disease has not shown progression after her last injection some 28 mo previously. Her age may be a contributing factor—54 y old at time of vaccine commencement. A Phase II randomized controlled trial found that vaccinated patients under 60 y of age received increased survival times relative to unvaccinated patients. On the other hand those over 60 y old had no significant differences in survival.5 Madam FNM’s antibody titers were unobtainable. This is a limitation of this report. However it would be interesting to measure her anti EGF titers. Longer lasting and larger antibody titers have been successfully produced by EGF booster inoculations in animal models.12 So far these attempts in humans have not elicited similar results15
Madam FNM’s side effect of back pain is unique to the authors. Successful treatment by antihistamine suggests that Madam FNM experienced an anaphylactoid reaction. These mediators can cause ureteric smooth muscle to spasm.18 This may explain her renal colic-like symptoms. However the real mechanism is unclear. Trials involving vaccines including Montanide ISA 51, hu-EGF and P64K protein have been known to cause Grade 2 reactogenicity reactions.19–21 These include some local inflammation and constitutional symptoms. While Montanide ISA 51 has been used extensively,22 experience with hu-EGF and P64K is relatively new. Data from the Phase III study may shed more light on this reaction.
Conclusion
The CIMAvax vaccine regime offers hope in significantly extending survival time to those who have an otherwise short life expectancy. Further research is important in uncovering side effects. As well, further experimentation in methods of vaccine composition and delivery may well improve the CIMAvax regime’s efficacy.
Author Contributions
Jian Cheng wrote the manuscript and Ratnavelu Kananathan was the physician overseeing Madam FNM’s treatment.
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/21744
References
- 1.Johnson DH. Chemotherapy for metastatic non-small-cell lung cancer--can that dog hunt? J Natl Cancer Inst. 1993;85:766–7. doi: 10.1093/jnci/85.10.766. [Comment Editorial] [DOI] [PubMed] [Google Scholar]
- 2.D’Addario G, Felip E, ESMO Guidelines Working Group Non-small-cell lung cancer: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2008;19(Suppl 2):ii39–40. doi: 10.1093/annonc/mdn081. [Practice Guideline] [DOI] [PubMed] [Google Scholar]
- 3.Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–50. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 4.González G, Crombet T, Neninger E, Viada C, Lage A. Therapeutic vaccination with epidermal growth factor (EGF) in advanced lung cancer: analysis of pooled data from three clinical trials. Hum Vaccin. 2007;3:8–13. doi: 10.4161/hv.3.1.3537. [DOI] [PubMed] [Google Scholar]
- 5.Neninger Vinageras E, de la Torre A, Osorio Rodríguez M, Catalá Ferrer M, Bravo I, Mendoza del Pino M, et al. Phase II randomized controlled trial of an epidermal growth factor vaccine in advanced non-small-cell lung cancer. J Clin Oncol. 2008;26:1452–8. doi: 10.1200/JCO.2007.11.5980. [DOI] [PubMed] [Google Scholar]
- 6.Lo HW, Hung MC. Nuclear EGFR signalling network in cancers: linking EGFR pathway to cell cycle progression, nitric oxide pathway and patient survival. Br J Cancer. 2006;94:184–8. doi: 10.1038/sj.bjc.6602941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirsch FR, Varella-Garcia M, Cappuzzo F. Predictive value of EGFR and HER2 overexpression in advanced non-small-cell lung cancer. Oncogene. 2009;28(Suppl 1):S32–7. doi: 10.1038/onc.2009.199. [DOI] [PubMed] [Google Scholar]
- 8.Neninger E, Verdecia BG, Crombet T, Viada C, Pereda S, Leonard I, et al. Combining an EGF-based cancer vaccine with chemotherapy in advanced nonsmall cell lung cancer. J Immunother. 2009;32:92–9. doi: 10.1097/CJI.0b013e31818fe167. [Clinical Trial] [DOI] [PubMed] [Google Scholar]
- 9.Ramos TC, Vinageras EN, Ferrer MC, Verdecia BG, Rupalé IL, Pérez LM, et al. Treatment of NSCLC patients with an EGF-based cancer vaccine: report of a Phase I trial. Cancer Biol Ther. 2006;5:145–9. doi: 10.4161/cbt.5.2.2334. [DOI] [PubMed] [Google Scholar]
- 10.García B, Neninger E, de la Torre A, Leonard I, Martínez R, Viada C, et al. Effective inhibition of the epidermal growth factor/epidermal growth factor receptor binding by anti-epidermal growth factor antibodies is related to better survival in advanced non-small-cell lung cancer patients treated with the epidermal growth factor cancer vaccine. Clin Cancer Res. 2008;14:840–6. doi: 10.1158/1078-0432.CCR-07-1050. [DOI] [PubMed] [Google Scholar]
- 11.Rodríguez PC, Rodríguez G, González G, Lage A. Clinical development and perspectives of CIMAvax EGF, Cuban vaccine for non-small-cell lung cancer therapy. MEDICC Rev. 2010;12:17–23. doi: 10.37757/MR2010.V12.N1.4. [Review] [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez PC, Gonzalez I, Gonzalez A, Avellanet J, Lopez A, Perez R, et al. Priming and boosting determinants on the antibody response to an Epidermal Growth Factor-based cancer vaccine. Vaccine. 2008;26:4647–54. doi: 10.1016/j.vaccine.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez G, Montero E, Leon K, Cohen IR, Lage A. Autoimmunization to epidermal growth factor, a component of the immunological homunculus. Autoimmun Rev. 2002;1:89–95. doi: 10.1016/S1568-9972(01)00015-5. [Review] [DOI] [PubMed] [Google Scholar]
- 14.González G, Crombet T, Catalá M, Mirabal V, Hernández JC, González Y, et al. A novel cancer vaccine composed of human-recombinant epidermal growth factor linked to a carrier protein: report of a pilot clinical trial. Ann Oncol. 1998;9:431–5. doi: 10.1023/A:1008261031034. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez G, Crombet T, Torres F, Catala M, Alfonso L, Osorio M, et al. Epidermal growth factor-based cancer vaccine for non-small-cell lung cancer therapy. Ann Oncol. 2003;14:461–6. doi: 10.1093/annonc/mdg102. [DOI] [PubMed] [Google Scholar]
- 16.González G, Lage A. Cancer vaccines for hormone/growth factor immune deprivation: a feasible approach for cancer treatment. Curr Cancer Drug Targets. 2007;7:229–41. doi: 10.2174/156800907780618310. [DOI] [PubMed] [Google Scholar]
- 17.National Institutes of Health. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. National Cancer Institute; 2009 [cited 2012 28/7/12]; Available from: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf
- 18.Brown SG, Mullins RJ, Gold MS. Anaphylaxis: diagnosis and management. Med J Aust. 2006;185:283–9. doi: 10.5694/j.1326-5377.2006.tb00619.x. [Review] [DOI] [PubMed] [Google Scholar]
- 19.Graham BS, McElrath MJ, Keefer MC, Rybczyk K, Berger D, Weinhold KJ, et al. AIDS Vaccine Evaluation Group Immunization with cocktail of HIV-derived peptides in montanide ISA-51 is immunogenic, but causes sterile abscesses and unacceptable reactogenicity. PLoS One. 2010;5:e11995. doi: 10.1371/journal.pone.0011995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pérez A, Dickinson F, Cinza Z, Ruíz A, Serrano T, Sosa J, et al. Safety and preliminary immunogenicity of the recombinant outer membrane protein P64k of Neisseria meningitidis in human volunteers. Biotechnol Appl Biochem. 2001;34:121–5. doi: 10.1042/BA20010029. [DOI] [PubMed] [Google Scholar]
- 21.Toledo H, Baly A, Castro O, Resik S, Laferté J, Rolo F, et al. A phase I clinical trial of a multi-epitope polypeptide TAB9 combined with Montanide ISA 720 adjuvant in non-HIV-1 infected human volunteers. Vaccine. 2001;19:4328–36. doi: 10.1016/S0264-410X(01)00111-6. [DOI] [PubMed] [Google Scholar]
- 22.Chiang CL, Kandalaft LE, Coukos G. Adjuvants for enhancing the immunogenicity of whole tumor cell vaccines. Int Rev Immunol. 2011;30:150–82. doi: 10.3109/08830185.2011.572210. [DOI] [PubMed] [Google Scholar]


