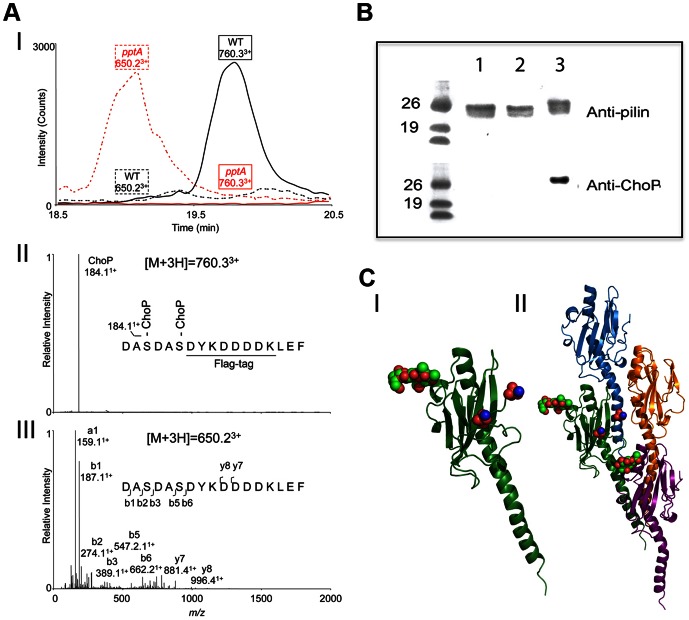
Figure 3. Analysis of site of ChoP addition in N. meningitidis C311#3.
(A) LC-MS/MS of trypsin-digested purified pilin showed that two ChoP residues are attached to Ser157 and Ser160 in WT C311#3. (I) Extracted ion chromatograms of ChoP-modified (m/z of 760.3) and unmodified pilin peptides (m/z of 650.2) in C311#3 WT and C311#3pptA purified pilin. (II) MS/MS of ChoP modified pilin peptides of C311#3 WT. The MS/MS of [M+3H]3+ signal at m/z of 760.3 suggested this peptide is modified with two ChoP, which has an ion mass of 184.1+ Da. (III) LC-ESI/MS analysis of trypsin-digested pilin from C311#3pptA lacked ChoP on pilin as indicated by the unmodified 155DASDASDYKDDDDKLEF170 peptide [M+3H]3+ ion at m/z of 650.2 (neutral mass of 1947.6 Da) and the absence of [M+3H]3+ ion at m/z 760.3. (B) Western Blot analysis of pilin from N. meningitidis C311#3 S157A/S160A mutant (no FLAG-tagged) demonstrates that ChoP is only present on Ser 157 and Ser 160. Lane 1 – C311#3S157A/S160A, lane 2 – C311#3pptA pilin, lane 3 – WT pilin. (C) Modelling of the pili-linked glycan (Ser63; red/green spheres) and ChoP (red/blue spheres) on the C-terminus of the pilin subunit (I) demonstrates their juxtaposition when adjacent pilin subunits are stack in the pili polymer (II).