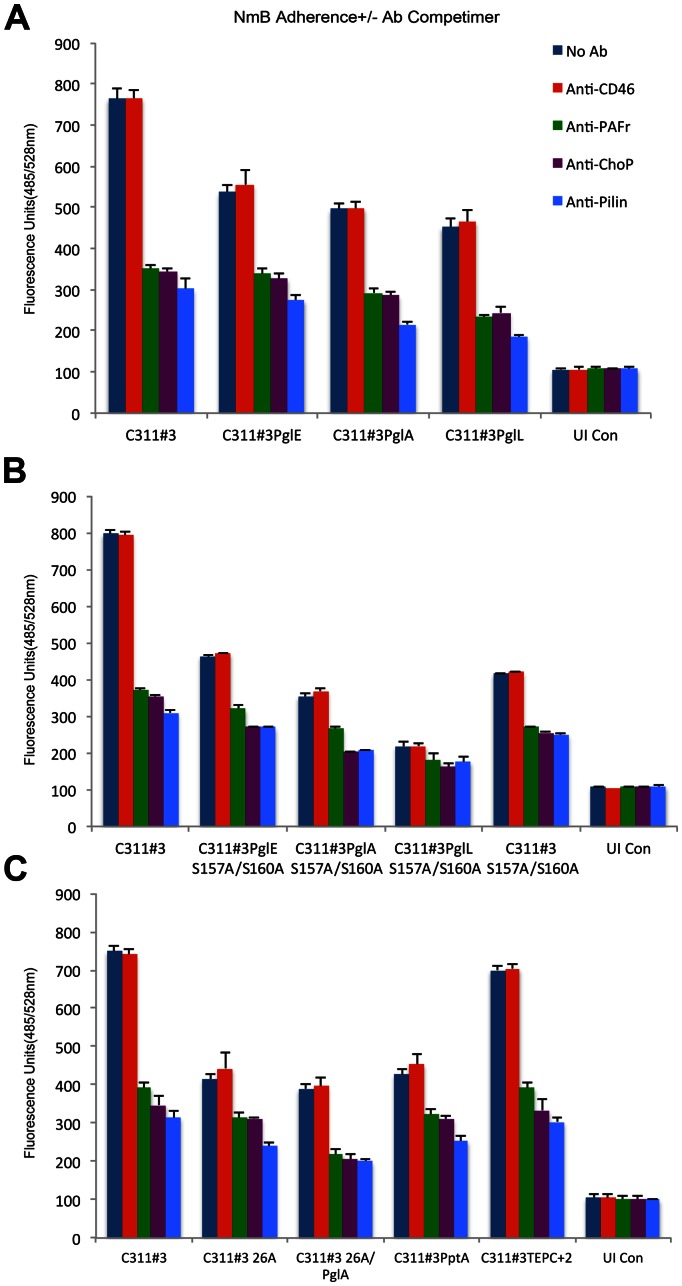
Figure 5. Cell association assays performed on a range of capsulate C311#3 mutants in the presence and absence of competimers.
A fluorometric adherence assay, as described in the text, was used to determine the contribution of ChoP and glycan pilin modifications to a pilus-PAFr interaction on 16HBE14 cells. Antibody competimers were omitted (dark blue bars) or included in the assay and comprised anti-CD46 (red bars), -PAFr (green bars), -ChoP (purple bars), and -pilin (light blue bars). Arbitrary fluorescence units (y-axis) were recorded 30 minutes post-challenge of 16HBE14 cells with GFP-expressing C311#3 WT and mutant strains, as noted. UI Con - uninfected (control) 16HBE14 cells; (Panel A) C311#3 (WT, trisaccharide, 2 ChoP), PglE (pglE mutant, disaccharide, 2 ChoP), PglA (pglA mutant, monosaccharide, 2 ChoP), PglL (pglL mutant, no glycan, 2 ChoP); (Panel B) S157A/S160A (C311#3 site-specific mutant with a serine to alanine conversion generated at amino acids 157 and 160; trisaccharide, no ChoP); (Panel C) 26A (strain C311#326A, C311#3 natural variant, trisaccharide, no ChoP), 26APglA (C311#326A pglA mutant, monosaccharide, no ChoP), PptA (pptA mutant, trisaccharide, no ChoP), TEPC+2 (C311#3 natural variant with a serine to alanine conversion at amino acid 68; trisaccharide, 2 ChoP, hyper-reactive to TEPC-15 antibody). Adherence was significantly (P≤ 0.0001) impaired by the inclusion (vs. the omission of an antibody competimer) of anti-PAFr, -ChoP, or -pilin antibody competimers to each infection assay, whereas no significant (P≥0.2338) difference was observed when anti-CD46 antibody was included to block the association of meningococci with 16HBE14 cells. These data provide strong evidence that the initial contact of meningococci with human airway cells occurs via a pilus-PAFr interaction in which both the pilin glycan and ChoP play important contributory roles.