Abstract
An efficient regeneration protocol for Sarcostemma acidum – an important medicinal plant has been established. Callus initiated from nodal explant on MS medium with 2.0 mg L−1 of NAA + additives. Callus initiated was subcultured on MS medium containing various concentrations of NAA or 2,4-D. Out of these combinations, MS medium +1.0 mg L−1 of NAA + additives was found to be effective for the multiplication of callus. Subculture was done after an interval of 20–22 days. For differentiation of callus BAP or Kinetin alone was found to be less effective. Maximum frequency of shoot regeneration recorded on MS medium +1.0 mg L−1 of BAP + 0.5 mg L−1 of Kinetin and 0.1 mg L−1 of NAA + additives. The in vitro differentiated shoots were excised and inoculated on 1/4 strength MS medium +2.0 mg L−1 of IBA + 0.02 % activated charcoal for in vitro rooting. Maximum response (90 %) was recorded on this medium. In vitro differentiated shoots were inoculated on autoclaved soilrite® after treatment with root inducing auxins. Ex vitro rooting in this plant species has been reported for the first time. Eighty five percent of the shoots rooted under ex vitro conditions. Both in vitro and ex vitro rooted plantlets were hardened in a green house.
Keywords: Adenine sulphate, Ex vitro, Hardening, In vitro, Micropropagation, Sarcostemma acidum
Introduction
Sarcostemma acidum (Roxb.), a xerophytic plant belongs to family Asclepiadaceae. Plant is locally known as Khir khimp, Khursani tanto, Art thor, Soma and Somavalli, in English- Moon plant and Moon creeper, in Hindi- Somlata. It is much branched, leafless straggling shrub, climbing on Euphorbia caducifolia haines on hills (Shetty and Singh 1993). It usually flowers in summer and rains. Fruiting is observed during October. Plant is mainly propagated through seeds in nature. Plant is medicinally important as dried stems are emetic, treatment of leprosy patients. Roots have been used in cases of snake bite and rabies (Verma et al. 2002). The root is taken as infusion in dog bites cases (Bhandari 1990). Plant is bitter, acrid, cooling, narcotic, emetic, antiviral and rejuvenating. Plant is useful in vitiated conditions of pitta, dipsia, hydrophobia, psychopathy and general dibility (Prajapati et al. 2003). In the Vedic literature it has been called Soma, a sacred plant. The juice of this plant is considered as the divine drink offered to gods, contemplated with medicinal efficacy, used as natural restorative for health that makes the consumer awakened and alert (Padhy and Dash 2004).
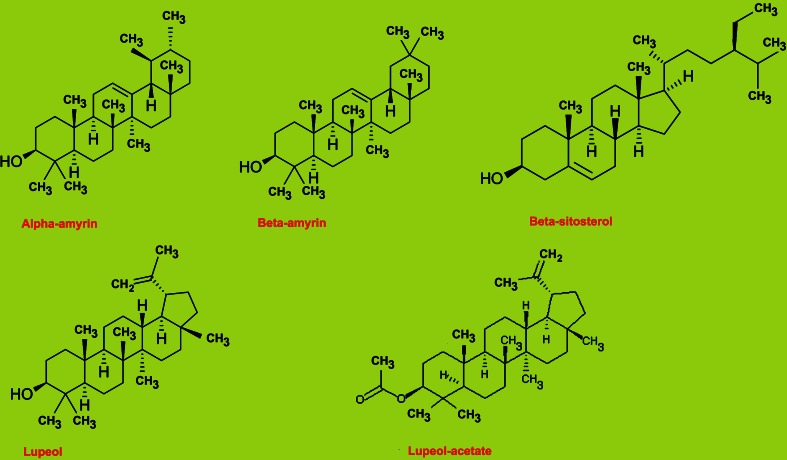
Plant contains many important phytochemicals like- malic acid, succinic acid, reducing sugars,α and β-amyrins, lupeol and lupeol-acetate and β-sitosterol (Fig. 1) (Prajapati et al. 2003). It has four lignans, sacidumlignans A–D (1–4), and two degraded lignan derivatives, sacidumols A (5) and B (6), (+)-pinoresinol, 9α-hydroxypinoresinol, perforatic acid, and peucenine-7-O-methyl ether, isolated from the ethanolic extract of the whole plant of Sarcostemma acidum. Among these Sacidumlignan A (1) showed moderate antimicrobial activities against two Gram-positive bacteria in vitro (Gan et al. 2005). The species of Sarcostemma are used by cultivators to extirpate white ants from sugar-cane fields. A bundle of twigs is put into trough from which the field is watered together with a bag of salt and water thus impregnated destroys the white ants without affecting the crop (Tiagi and Kshetrapal 1989). Micropropagation protocol has not yet been defined for this rare medicinal species before. The know-how generated during the present investigation can be used for propagation and conservation of S. acidum.
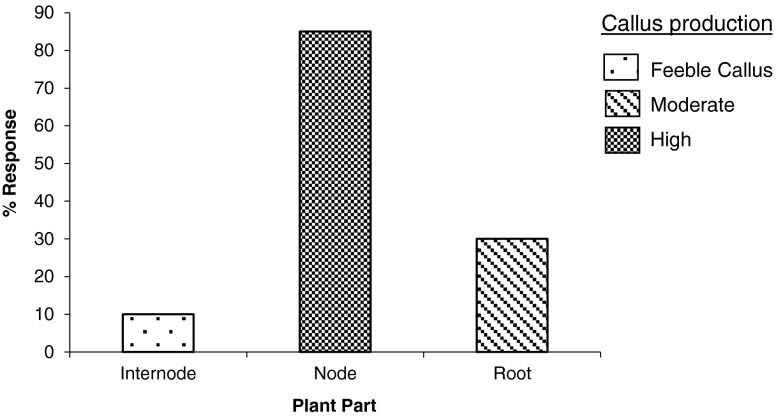
Graph 1.
Percentage response of explants of S. acidum on MS medium +2.0 mg L−1 NAA + additives
Material and methods
Establishment of cultures
Plants used as source of explants for establishment of cell cultures, were collected from Machia Safari Park, Jodhpur (Fig. 2a). These were maintained and managed in a green house at plant biotechnology unit, JNVU, Jodhpur. Nodal, internodal segments and roots were evaluated as explants to establish the cultures. These were surface sterilized with 0.1 % HgCl2 for 4–5 min and were washed several times with autoclaved water. Surface sterilized explants were inoculated on MS medium (Murashige and Skoog 1962) supplemented with NAA (0.5, 1.0, 1.5, 2.0, 3.0, 4.0 and 5.0 mg L−1) or 2,4-D (1.0, 2.0, 3.0, 4.0 and 5.0 mg L−1) alongwith additives (25.0 mg L−1 each of citric acid and adenine sulphate +50.0 mg L−1 ascorbic acid) and incubated under diffused light (20–30 μmolm−2s−1 SFP ) at 26 ± 2 °C and RH 40–60 %. Commercial grade sugar and agar-agar were used for preparation of artificial culture medium.
Fig. 1.
Important active principles from S. acidum
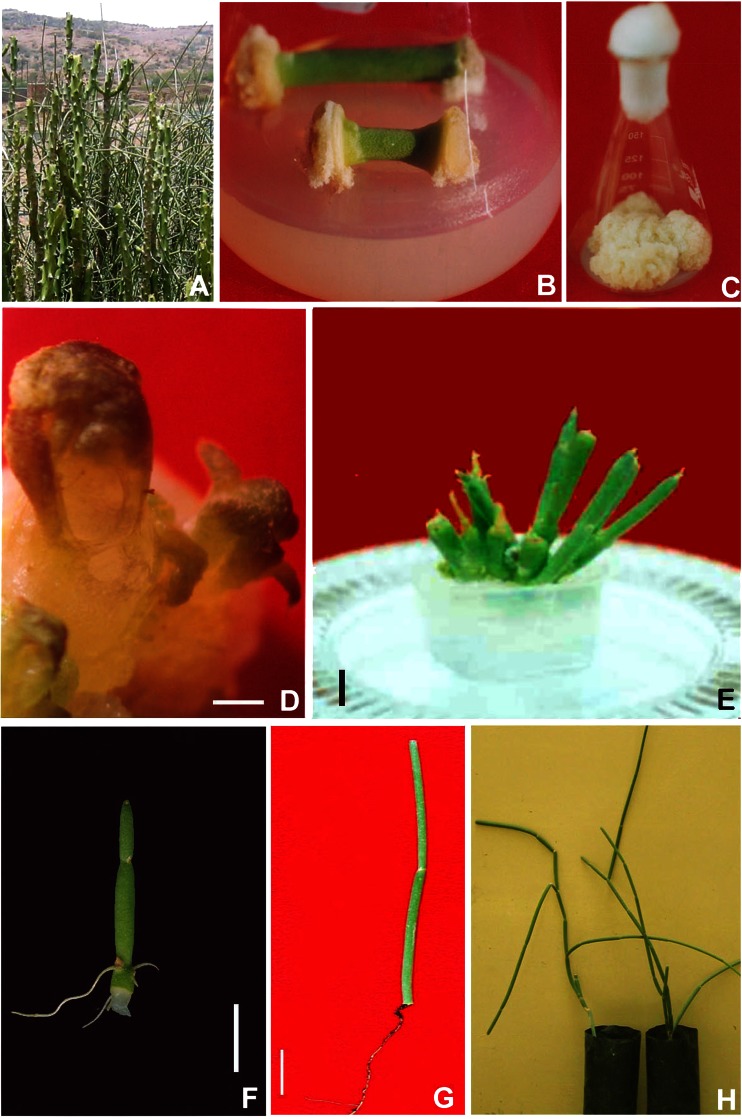
Fig. 2.
Plantlet regeneration in S. acidum. aS. acidum climbing on E. caducifolia haines. b Callus initiation from nodal regions on MS medium +2.0 mgl−1 of NAA + additives. c Amplification of callus on MS medium +1.0 mg/l of NAA + additives. d Microphotograph of regeneration of shoot from callus of S. acidum (5X; bar = 1 mm). e Direct regeneration of shoots from callus cultures on MS medium +1.0 mg/l BAP + 0.5 mg/l Kinetin + 0.1 mg/l NAA + additives (bar = 5 mm). f In vitro rooting of differentiated shoot on 1/4 MS medium +2.0 mg/l IBA + 0.02 % activated charcoal (bar = 5 mm). g Ex vitro rooted shoot of S. acidum treated with 200 mg/l of IBA (bar = 10 mm). h Hardened plants of S. acidum transferred to polybags in green house
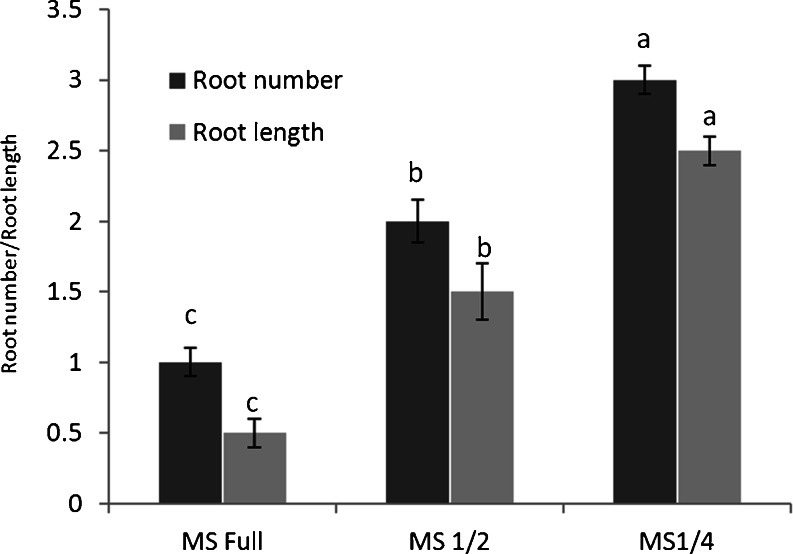
Graph 2.
Effect of different strengths of MS basal salts concentrations on in vitro rooting
Multiplication of callus
The cultures were further multiplied by subculture on MS + 2,4-D (0.5, 1.0, 2.0 and 3.0 mg L−1) or NAA (0.5, 1.0, 2.0, 3.0, 4.0 and 5.0 mg L−1) along with additives. Callus was regularly subcultured in 250 ml conical flasks and were incubated under 20–30 μmolm−2s−1 SFP at 28 ± 2 °C temperature in growth room.
Plant regeneration through organogenesis
The callus was transferred on to PGR free-MS medium (used as a control) and MS medium containing different concentrations (1.0, 2.0, 3.0, 4.0 and 5.0 mg L−1) of cytokinins (BAP or Kinetin). Callus cultures were also inoculated on semisolid MS medium containing different concentrations and combinations of cytokinins (0.5, 1.0, 1.5 and 2.0 of BAP + Kinetin 0.5 mg L−1) +0.1 mg L−1 NAA along with additives for differentiation of callus. The cultures were incubated at high light intensity (30–40 μmolm−2s−1 SFP) at 28 ± 2 °C temperature.
Rooting of shoots
The shoots regenerated through callus cultures were subjected to in vitro and ex vitro rooting approaches. In vitro produced shoots were inoculated on to half, one fourth and full strength MS medium +0.02 % activated charcoal containing various concentrations (1.0, 2.0, 3.0, 4.0 and 5.0 mg L−1) of root-inducing auxins (IBA and NOA). These were incubated at 28 ± 2 °C under diffused light conditions. In vitro rooted shoots were removed from culture vessels and washed with autoclaved water to remove adhered agar. The rooted shoots were inoculated in bottles containing autoclaved soilrite® (a mix of perlite with peat moss and exfoliated vermiculite purchased from Kel Perlite, Bangalore, India) and moistened with one-fourth strength of MS macro salts solution. These bottles were capped and kept near pad section of green house. Healthy shoots were harvested and treated with various concentrations (50.0, 100.0, 200.0, 300.0, 400.0 and 500.0 mg L−1) of auxins (IBA or NOA) for different time durations (1, 2, 3, 4 and 5 min) for ex vitro rooting. These were inoculated in soilrite and moistened with 1/4 strength MS basal salts and kept under green house conditions for hardening.
Acclimatization and pot transfer
Both in vitro and ex vitro rooted shoots were hardened under regimes of temperature and humidity in the green house. These hardened plantlets were transferred to poly-bags containing Sand: Clay: FYM in ratio 3:1:1 and were kept in green house initially and then transferred to nursery.
Protein profiling by SDS-PAGE
Weighed amount of callus was grinded in 0.2 M phosphate buffer of pH 6.0 (0.1 g/ml) and centrifuged at 10,000 rpm at 4 °C for 40 min in refrigerated centrifuge (SIGMA 2K15). The supernatant was used for further analysis and the pellet was discarded. Total soluble protein content was determined by the method described by Bradford (1976). To the supernatant equal volume of Laemmli’s buffer (Laemmli 1970) was added. The solution was vortexed and heated at 100 °C for 90 s. The samples prepared were loaded in the wells of gel. Proteins were resolved on 12.5 % resolving gel. Vertical Polyacrylamide Gel Electrophoresis was performed using a model of ATTO AE6530 mPAGE (ATTO Corporation, Japan) on slab gels (120 × 102 × 7 mm) for 1 mm thick hand-cast gel. Power supply for running the gel was given by ATTO CONSTAPOWER 3000 AE-8800. The gel was subjected to 40mA constant current for 1 h at a variable voltage and power at low temperature of 10 °C in a cold room (Blue Star). The gel was stained with silver nitrate solution (Wray et al. 1981). The gel was analyzed using Syngene Gel Documentation System. Gene Tool Software was used to prepare analysis report of proteins separated by PAGE.
Statistical analysis
The experiments were laid down according to Randomized Block Design (RBD) for single factor experiments (Compton and Mize 1999). The experiments were repeated three times with a minimum of ten replicates per treatment. The data was recorded at regular interval of 15 days. The data was statistically analyzed for Duncan’s Multiple range test at P < 0.05. Results articulated in the tables are in mean ± SD.
Results and discussion
Establishment of callus culture
The nodal region was found to be suitable explant for establishment of cell cultures. Roots responded moderately while internodes produced feeble callus (Graph. 1). Callus initiation started after 10–15 days of inoculation. White, granular and fast growing callus was obtained. Callus produced from roots was soft, fragile and brown. Callus derived from node on MS + 2.0 mg L−1 of NAA, was fast growing (Fig. 2b). The callus produced on medium containing low (0.5, 1.0 and 1.5 mg L−1) or high (3.0, 4.0 and 5.0 mg L−1) concentrations of NAA was slow growing, compact and non regenerative. The cultures produced on medium with 2,4-D were brown and slow growing (Table 1). Studies have shown effect of mineral nutrients on plant morphogenesis in culture conditions (Ramage and Williams 2002). Selection of appropriate explant material is a crucial step for establishment of in vitro cell cultures (Rathore et al. 2013).
Table 1.
Effect of concentrations of auxins (2,4-D and NAA) on callus induction in Sarcostemma acidum on MS medium + additives
| Name of PGR | Concentration of PGR (mg L−1) | Callus intensity | Remarks |
|---|---|---|---|
| Control | 0.0 | − | No response |
| 2,4-D | |||
| 0.5 | − | No response | |
| 1.0 | + | Slow growing callus | |
| 1.5 | + | Slow growing, watery callus | |
| 2.0 | ++ | Slow growing and fragile callus | |
| 3.0 | ++ | Brown and fragile callus | |
| 4.0 | ++ | Limited growth, brown, fragile callus | |
| 5.0 | + | Explant become brown, Poor growth | |
| NAA | |||
| 0.5 | + | Slow response, non-regenerative callus | |
| 1.0 | + | Non-regenerative callus | |
| 1.5 | + | Non-regenerative callus | |
| 2.0 | +++ | White, granular and fast growing callus | |
| 3.0 | +++ | Fast-growing, fragile callus | |
| 4.0 | ++ | Slow growing and fragile callus | |
| 5.0 | ++ | Browning of callus |
+ = Slow; ++ = Moderate; +++ = High
Effects of PGRs on multiplication of callus cultures
MS medium containing 1.0 mg L−1 of NAA + additives was found to be effective for the multiplication of cell cultures (Table 2) (Fig. 2c). Growth of the callus was slow on MS medium containing 0.5 or 1.0 mg L−1 of 2,4-D. On higher concentrations (2.0 and 3.0 mg L−1), the growth of the callus was poor and the callus turned brown. The cultures of S. acidum were thus maintained on MS medium +1.0 mg L−1 NAA. Production of cells takes a number of forms and it appears that it is influenced by a diversity of factors, some of which can be controlled during the culture progression, and some of which are yet indeterminate (Gretchen 2005; Rathore and Shekhawat 2009).
Table 2.
Effect of concentrations of growth regulators on multiplication of callus cultures of S. acidum on MS medium + additives
| Name of PGR | Concentration of PGR (mg L−1) | Callus intensity | Remarks |
|---|---|---|---|
| Control | 0.0 | + | Slow growing callus |
| 2,4-D | 0.5 | + | Slow growing glassy callus |
| 1.0 | ++ | Slow growing and fragile callus | |
| 2.0 | + | Brown and fragile callus | |
| 3.0 | + | Slow growing, brown, fragile callus | |
| NAA | 0.5 | + | Slow growing, non-regenerative callus |
| 1.0 | +++ | White, fast growing granular callus | |
| 2.0 | ++ | Granular and fast growing callus | |
| 3.0 | ++ | Fast-growing, fragile callus | |
| 4.0 | + | Fast-growing, fragile callus | |
| 5.0 | + | Slow-growing, brown callus |
+ = Slow; ++ = Moderate; +++ = High
Differentiation of callus
The cell mass cultured in vitro exhibited tendency to differentiate. On medium supplemented with BAP or Kinetin, there was very low differentiation of the callus culture (Table 3). The cultures differentiated through organogenesis and produced multiple shoots (5–6 shoots per vessel) on MS medium +1.0 mg L−1 of BAP + 0.5 mg L−1 of Kinetin and 0.1 mg L−1 of NAA and additives (Table 4) (Fig. 2d & e). The shoot formation is affected by number of intrinsic and extrinsic factors (Rathore and Shekhawat 2009; Rathore et al. 2013). Skoog and Miller (1957) showed that the developmental fate of regenerating tobacco tissues in culture could be directed by PGRs. The ability to direct the course of development by two simple plant hormones has intrigued plant biologist for years (Che et al. 2006). Much has been learned in the past few years about cytokinin and auxin signaling, but less is known about developmental events downstream. Programs of callus and organ development are associated with gene expression (Verpoorte and Alfermann 2000).
Table 3.
Effects of different types and concentrations of cytokinins on organogenesis from callus of S .acidum on MS medium + additives
| Name of PGR | Concentration of PGR (mg L−1) | Shoot number (mean ± SD) | Shoot length (cm.) (mean ± SD) |
|---|---|---|---|
| Control | 0.0 | 0.5 ± 0.52d | 0.62 ± 0.65f |
| BAP | 1.0 | 2.1 ± 0.73b | 2.20 ± 0.29b |
| 2.0 | 2.5 ± 0.52a | 2.42 ± 0.28a | |
| 3.0 | 1.5 ± 0.52bc | 1.51 ± 0.19c | |
| 4.0 | 1.3 ± 0.48c | 1.30 ± 0.16d | |
| 5.0 | 1.1 ± 0.31c | 0.90 ± 0.42e | |
| Kinetin | 1.0 | 1.7 ± 0.67a | 2.16 ± 0.27a |
| 2.0 | 1.5 ± 0.52ab | 1.78 ± 0.22b | |
| 3.0 | 1.3 ± 0.48b | 1.39 ± 0.16c | |
| 4.0 | 0.9 ± 0.31bc | 0.88 ± 0.41d | |
| 5.0 | 0.7 ± 0.48c | 0.29 ± 0.23e |
Means in every column followed by identical letter are not significantly different at P < 0.05
Table 4.
Effects of BAP concentrations on organogenesis from callus of S .acidum on MS medium + 0.5 mg L−1 Kinetin + 0.1 mg L−1 NAA + additives
| Name of PGR | Concentration of PGR (mg L−1) | Shoot number (mean ± SD) | Shoot length (cm.) (mean ± SD) |
|---|---|---|---|
| Control | 0.0 | 0.5 ± 0.52e | 0.62 ± 0.65e |
| BAP | 0.5 | 3.1 ± 0.87c | 2.35 ± 0.49b |
| 1.0 | 5.1 ± 0.87a | 3.87 ± 0.31a | |
| 1.5 | 3.6 ± 1.07b | 2.07 ± 0.48c | |
| 2.0 | 2.5 ± 0.52d | 1.41 ± 0.21d |
Rooting of in vitro regenerated shoots
The shoots produced in vitro, rooted the best on 1/4 strength MS salts +2.0 mg L−1 of IBA + 0.02 % of activated charcoal (Fig. 2f; Graph 2). Activated charcoal not only adsorbs growth regulators, but it also adsorbs substances presumed to be harmful like phenolics, oxidized phenolics and gases like ethylene and methane. These are substances that should be avoided or eliminated from in vitro environment to maintain normal growth and development (Thomas 2008). About 90 % shoot rooted on this medium and each shoot produced 3–4 roots (length 2–3 cm.). Rooting occurred after 15–20 days of inoculation. IBA was found effective for the root induction from shoots (Table 5) (Rathore and Shekhawat 2009). Group of genes serve as molecular signatures of the different developmental process (Rubio et al. 2009; Shimizu-Sato et al. 2009). Regenerating tissues can be directed along different developmental pathways by transferring to the appropriate organ induction medium (Kuppusamy et al. 2009). Ex vitro rooting has not been reported in this plant (Thomas and Shankar 2009). Eighty five percent of the shoots rooted under ex vitro conditions when treated with 200 mg L−1 of IBA for 5 min (Table 6) (Fig. 2g). From each shoot 1–2 roots of length 5–6 cm. differentiated after 20–25 days of inoculation. NOA was found to be less effective for the ex vitro rooting in the shoots of S. acidum. Ex vitro rooting is more economical as hardening and acclimation process occurs simultaneously. IBA proved to better auxin for rooting of shoots both in vitro and ex vitro conditions (Rathore et al. 2013).
Table 5.
Effect of concentrations of auxins on in vitro root induction from shoots on 1/4 strength MS medium + 0.02 % activated charcoal
| Name of PGR | Concentration of PGR (mg L−1) | % Response | Root number (mean ± SD) | Root length (cm.) (mean ± SD) |
|---|---|---|---|---|
| Control | 0.0 | 0 | 0.0 ± 0.0e | 0.00 ± 0.00d |
| IBA | 1.0 | 70 | 2.2 ± 0.63b | 1.93 ± 0.35b |
| 2.0 | 90 | 3.2 ± 0.63a | 2.38 ± 0.25a | |
| 3.0 | 90 | 2.1 ± 0.56b | 1.59 ± 0.19c | |
| 4.0 | 85 | 1.7 ± 0.67c | 1.25 ± 0.14c | |
| 5.0 | 70 | 1.4 ± 0.51d | 0.94 ± 0.37c | |
| NOA | ||||
| 1.0 | 45 | 0.6 ± 0.51c | 0.59 ± 0.54c | |
| 2.0 | 55 | 0.9 ± 0.56bc | 1.06 ± 0.59b | |
| 3.0 | 60 | 1.2 ± 0.42ab | 1.41 ± 0.22a | |
| 4.0 | 75 | 1.4 ± 0.51a | 0.89 ± 0.42b | |
| 5.0 | 70 | 0.6 ± 0.51c | 0.34 ± 0.30d |
Means in every column followed by identical letter are not significantly different at P < 0.05
Table 6.
Effect of concentrations of IBA and NOA treatment on ex vitro rooting of shoots of Sarcostemma acidum
| Name of PGR | Concentration of PGR (mg L−1) | % Response | Root number (mean ± SD) | Root length (cm.) (mean ± SD). |
|---|---|---|---|---|
| Control | 0.0 | 0 | 0.00 ± 0.00e | 0.00 ± 0.00g |
| IBA | 50.0 | 40 | 0.7 ± 0.48d | 1.64 ± 1.20e |
| 100.0 | 70 | 1.1 ± 0.31c | 4.02 ± 0.53b | |
| 200.0 | 85 | 2.5 ± 0.52a | 5.81 ± 0.62a | |
| 300.0 | 80 | 1.6 ± 0.69b | 3.79 ± 0.52c | |
| 400.0 | 70 | 1.1 ± 0.31c | 2.15 ± 0.53d | |
| 500.0 | 70 | 0.7 ± 0.48d | 0.73 ± 0.60f | |
| NOA | 50.0 | 30 | 0.2 ± 0.42e | 0.23 ± 0.51e |
| 100.0 | 40 | 0.6 ± 0.51c | 0.91 ± 0.80c | |
| 200.0 | 55 | 1.1 ± 0.56b | 1.94 ± 0.82b | |
| 300.0 | 60 | 1.5 ± 0.52a | 2.59 ± 0.51a | |
| 400.0 | 50 | 1.0 ± 0.47b | 1.01 ± 0.89cd | |
| 500.0 | 45 | 0.5 ± 0.52d | 0.51 ± 0.59de |
Means in every column followed by identical letter are not significantly different at P < 0.05
Hardening and soil transfer of in vitro regenerated plantlets
Both in vitro and ex vitro rooted plantlets were hardened in green house by gradual loosening of caps of bottles and shifted away from pad section (temperature 22–24 °C and high RH i.e.80 %) gradually towards fan section (high temperature 28–30 °C and low RH i.e. 40 %). These plants were transferred from glass bottles to black poly-bags after 45 days and kept near fan section in green house. Hardened plants (after 60 days) from green house were transferred to nursery (Fig. 2h). The process of acclimation is essential to tissue culture raised plants as they are sensitive and doesn’t withstand the harsh conditions prevailing in the in situ conditions. Before transplanting them they have to be properly hardened for survival in field conditions (Rathore and Shekhawat 2009). Acclimatization of tissue-cultured plants has been subject of reviews (Pospisilova et al. 1999). Economic feasibility of any protocol is important in any industry. The use of commercial grade sugar, gelling agent and ex vitro rooting of plants has reduced the cost of production, which will definitely have an impact on the acceptance of this protocol.
Protein profiling
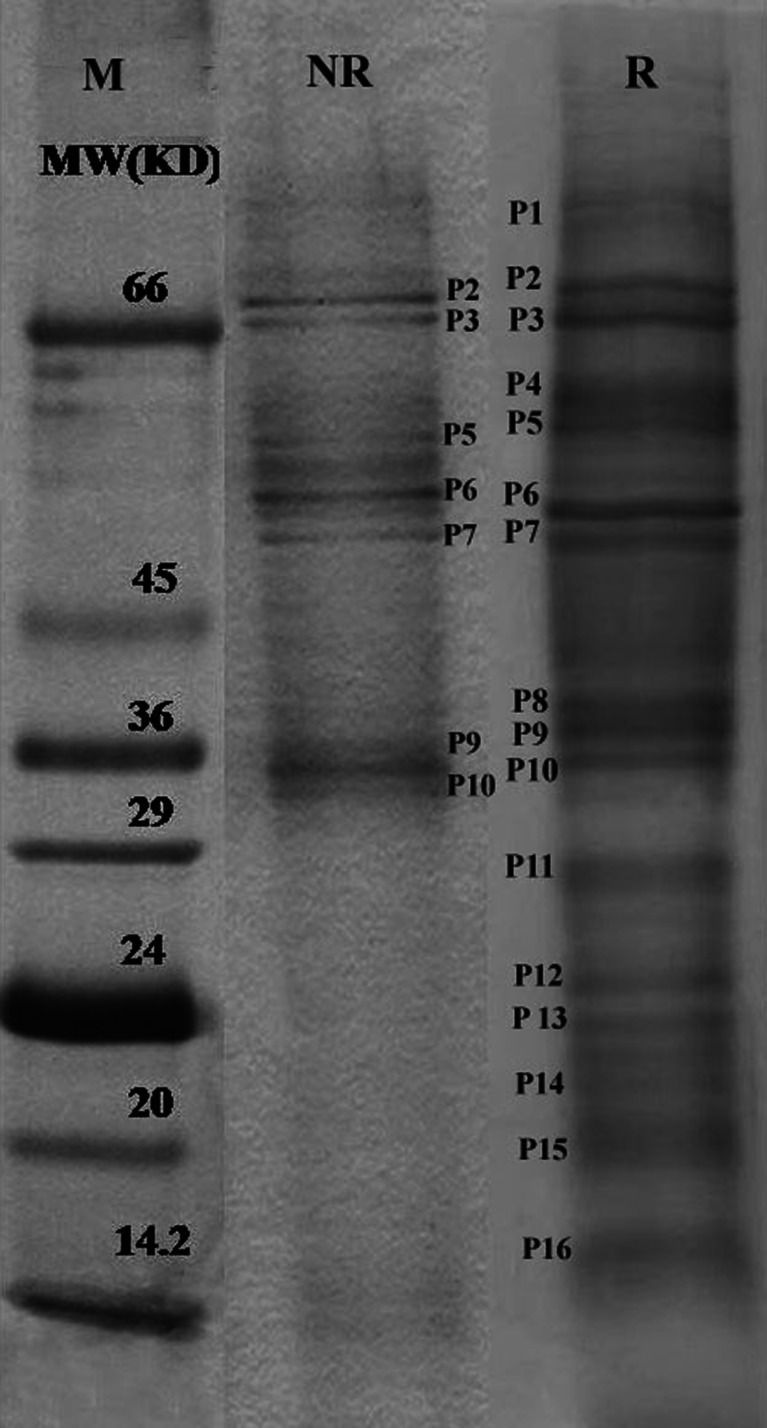
Protein profile of both the regenerative and non-regenerative callus of S. acidum as resolved on SDS-PAGE revealed a total number of 16 protein bands in regenerative and only 7 number of bands in non-regenerative callus (Fig. 3). Track 1 represents standard lane of polypeptides of known molecular weight (Marker lane), Track 2 represents proteins of non regenerative cultures and third lane is for regenerative culture. Proteins of molecular weight of 68, 45, 38 and 27-14 kD were present in the regenerative callus. Polypeptides of P2, P3, P5, P6, P7 and P9 in the profile showed intensity variation in both the types of cultures. Alongside the developmental stages, exogenous or endogenous signals, regulation of metabolic processes either by genes or enzymes, and their transport also play an important role in expression (Verpoorte and Alfermann 2000). It seems from the profile that the expression of low molecular weight proteins (<24 kD) is more in regenerative cultures and they have key role in organogenesis in S. acidum.
Fig. 3.
SDS-PAGE profile of regenerative and non-regenerative cultures of S. acidum ( M = marker, R = regenerative and NR = non regenerative)
Acknowledgements
We gratefully acknowledge the financial assistance provided by University Grants Commissions (UGC) of India and the Department of Science and Technology (DST), Govt. of India under SAP (Special Assistance Programme) and FIST (Infrastructure Development in Science and Technology) schemes sanctioned at the Department of Botany. The fundamental laboratory and greenhouse infrastructure used for research work has been established as Regional Micropropagation Unit for Arid regions with main funds from Department of Biotechnology (DBT), Govt. of India under Networking programmes on micropropagation.
Abbreviations
- BAP
6-Benzylaminopurine
- IAA
Indole-3 acetic acid
- IBA
Indole-3 butyric acid
- Kin
Kinetin
- MS
Murashige and Skoog medium
- NAA
α-Naphthalene acetic acid
- PGR
Plant growth regulator
- RH
Relative humidity
- SFP
Spectral flux photon
References
- Bhandari MM. Flora of Indian desert. Jodhpur: MPS Repros; 1990. p. 202. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of proteins of utilizing the principles of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Che P, Lall S, Nettleton D, Howell SH. Gene expression programme during shoot, root and callus development in Arabidopsis tissue culture. Plant Physiol. 2006;141:620–637. doi: 10.1104/pp.106.081240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton ME, Mize CW. Statistical considerations for in vitro research: I -Birth of an idea to collecting data. In Vitro Cell Dev Biol-Plant. 1999;35:115–121. [Google Scholar]
- Gan LS, Yang SP, Fan CQ, Yue JM. Lignans and their degraded derivatives from Sarcostemma acidum. J Nat Prod. 2005;68(2):221–225. doi: 10.1021/np049681s. [DOI] [PubMed] [Google Scholar]
- Gretchen V. How does a single somatic cell become a whole plant. Science. 2005;309:86. doi: 10.1126/science.309.5731.86. [DOI] [PubMed] [Google Scholar]
- Kuppusamy KT, Walcher CL, Nemhauser Cross-regulatory mechanisms in hormone signaling. Plant Mol Biol. 2009;69:375–381. doi: 10.1007/s11103-008-9389-2. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Clevage of structural proteins during the assembly of head of bacteriophage T4. Nature. 1970;220:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- Padhy S, Dash SK. The Soma drinker of ancient India: an ethno-botanical retrospection. J Hum Ecol. 2004;15(1):19–26. [Google Scholar]
- Pospisilovia J, Ticha I, Kadleeek P, Haisel D, Plzakova S. Acclimatization of micropropagated plants to ex vitro conditions. Biol Plant. 1999;42:481–497. doi: 10.1023/A:1002688208758. [DOI] [Google Scholar]
- Prajapati NS, Purohit SS, Sharma AK, Kumar T. A handbook of medicinal plants. India: Agrobios; 2003. p. 461. [Google Scholar]
- Ramage CM, Williams RR. Inorganic nitrogen requirements during shoot organogenesis in tobacco leaf discs. J Exp Bot. 2002;53:1437–1443. doi: 10.1093/jexbot/53.373.1437. [DOI] [PubMed] [Google Scholar]
- Rathore MS, Shekhawat NS. Micropropagation of Pueraria tuberosa (Roxb. Ex Willd.) and determination of puerarin content in different tissues. Plant Cell Tiss Organ Cult. 2009;99:327–334. doi: 10.1007/s11240-009-9608-9. [DOI] [Google Scholar]
- Rathore MS, Rathore MS, Shekhawat NS (2013) Ex vivo implications of phytohormones on various in vitro responses in Leptadenia reticulata (Retz.) Wight.& Arn.-An endangered plant. Environ Exp Bot 86:86–93. doi:10.1016/j.envexpbot.2010.05.009
- Rubio V, Bustos R, Irigoyen ML, Cardona-López X, Rojas-Triana M, Paz-Ares Plant hormones and nutrient signaling. Plant Mol Bio. 2009;69:361–373. doi: 10.1007/s11103-008-9380-y. [DOI] [PubMed] [Google Scholar]
- Shetty BV, Singh V. Flora of India Series-2: Flora of Rajasthan. Culcutta: Botanical Survey of India; 1993. p. 485. [Google Scholar]
- Shimizu-Sato S, Tanaka M, Mori H. Auxin-cytokinin interactions in the control of shoot branching. Plant Mol Bio. 2009;69:429–435. doi: 10.1007/s11103-008-9416-3. [DOI] [PubMed] [Google Scholar]
- Skoog F, Miller CO. Chemical regulation of growth and organ formation in plant tissue cultured in vitro. Symp Soc Exp Biol. 1957;XI:118–131. [PubMed] [Google Scholar]
- Thomas TD. The role of activated charcoal in plant tissue culture. Biotechnol Adv. 2008;26:618–631. doi: 10.1016/j.biotechadv.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Thomas TD, Shankar S. Multiple shoot induction and callus regeneration in Sarcostemma brevistigma Wight & Arnott, a rare medicinal plant. Plant Biotechnol Rep. 2009;3:67–74. doi: 10.1007/s11816-008-0076-1. [DOI] [Google Scholar]
- Tiagi YD, Kshetrapal S. An introduction to the taxonomy of angiosperms. Jaipur: Ramesh Book Depot; 1989. p. 299. [Google Scholar]
- Verma PK, Sharma A, Mathur A, Sharma P, Gupta RS, Joshi SC, Dixit VP. Effect of Sarcostemma acidum stem extract on spermatogenesis in male albino rats. Asian J Androl. 2002;4(1):43–47. [PubMed] [Google Scholar]
- Verpoorte R, Alfermann AW. Metabolic engineering of plant secondary metabolism. The Netherlands: Kluwer Academic Publishers; 2000. [Google Scholar]
- Wray W, Boulikas T, Wray VP, Hancock R. Silver staining in proteins in polyacrylamide gels. Anal Biochem. 1981;118:197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]