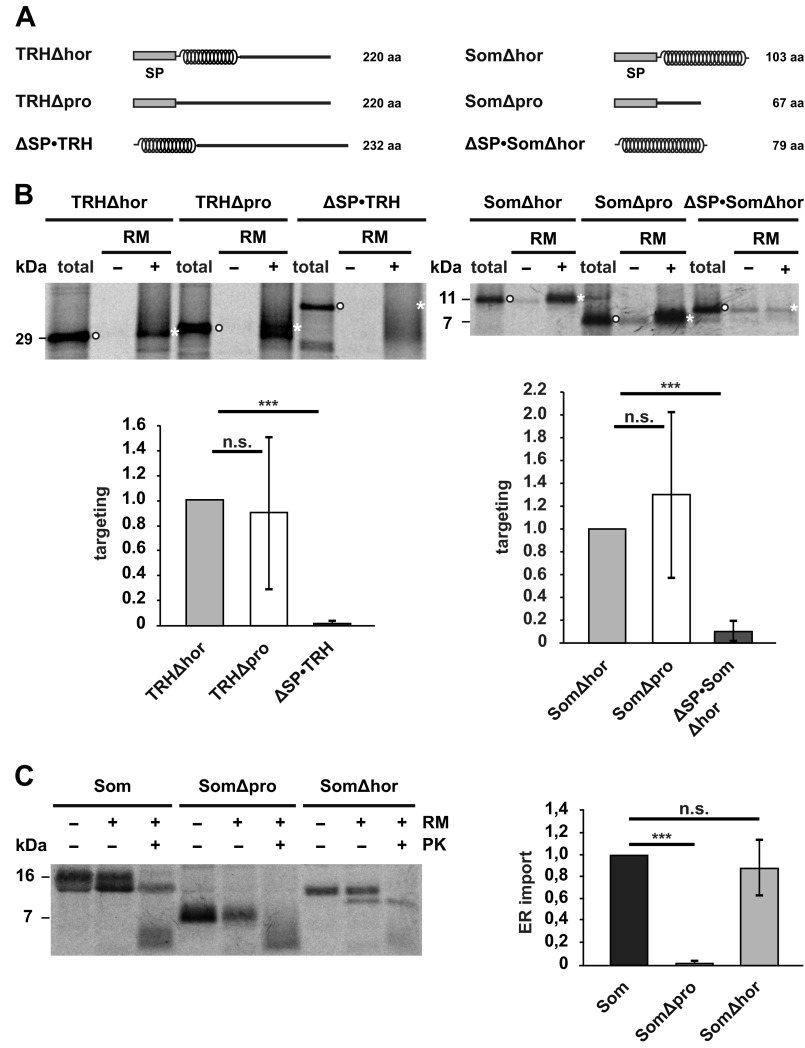
FIGURE 4.
α-Helical domains promote translocation of the nascent chain into the ER lumen. A, schematic representation of the constructs used. α-Helical structure is indicated by helices; intrinsic disorder is indicated by a straight line. SP, signal peptide. B, intrinsically disordered nascent chains are co-translationally targeted to ER membranes in vitro. Truncated mRNAs lacking a stop codon were translated in vitro using rabbit reticulocyte lysate supplemented with l-[35S]methionine in the presence (+) or absence (−) of rough RM. Targeted nascent chains were pelleted by centrifugation over a sucrose cushion for 3 min at 50,000 × g. In parallel, polysomes of translation reactions without microsomes were sedimented by centrifugation for 30 min at 50,000 × g (total). Samples were analyzed by SDS-PAGE and autoradiography. Targeting efficiency was determined by quantifying the band intensities of the respective constructs in the +RM fraction (white asterisks) relative to the amount present in the total fraction (white dots). Data represent mean ± S.E. (error bars) of at least three independent experiments. Targeting efficiency of the Δhor mutants was set as 1. ***, p < 0.0005; n.s., not significant. C, an intrinsically disordered protein is not imported into microsomes in vitro. Left panel, indicated constructs were translated in vitro using a rabbit reticulocyte lysate supplemented with l-[35S]methionine in the presence (+) or absence (−) of RM. After translation, samples were treated with proteinase K (+PK) or left untreated (−PK) and analyzed by SDS-PAGE and autoradiography. Right panel, import into microsomes was determined by quantifying the ratio between the signal of the proteinase K-protected fraction (+RM, +PK) to the total protein signal in the presence of microsomes (+RM, −PK). The relative amount of proteinase K-resistant wild type somatostatin was set as 1. Data represent mean ± S.E. (error bars) of at least three independent experiments. ***, p < 0.0005; n.s., not significant. The lower molecular smear (<7 kDa) present in the fractions treated with proteinase K (+PK) represents nonspecific degradation products that also appear after proteinase K digestion in the absence of microsomal membranes (data not shown).