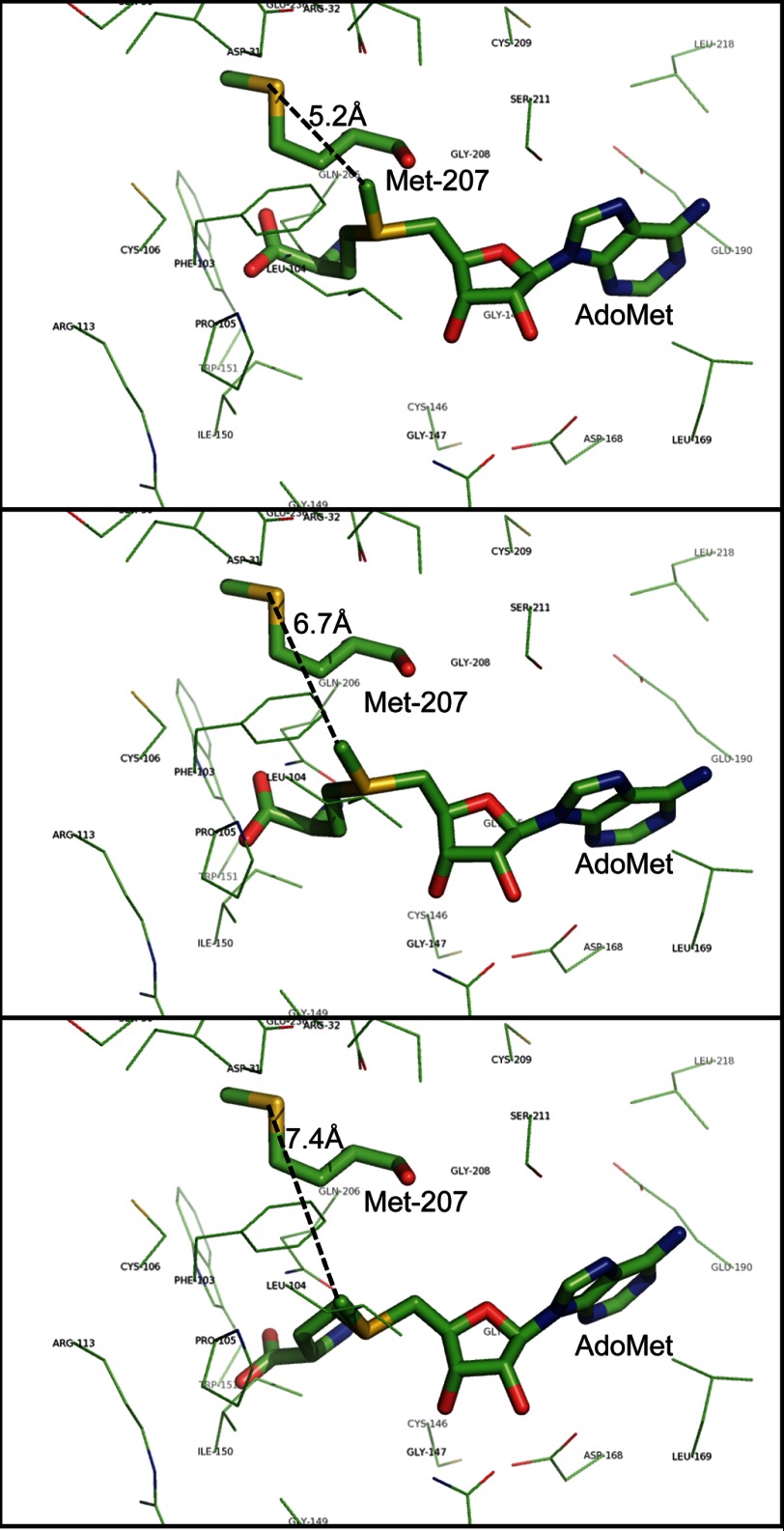
FIGURE 11.
Modeling of the LaeA structure suggests that the sulfur atom of methionine 207 is close to the position of the transferable methyl group of bound AdoMet. The three-dimensional structure of full-length LaeA was based on the primary sequence (UniProt accession number C8VQG9) and derived from Phyre2 modeling; the position of the ligand AdoMet was modeled by 3DLigandSite. The positions of three possible conformations of AdoMet are shown that encompass the extent of the eight different ligand structures obtained. LaeA side chain residues within 4 Å of AdoMet are shown as thin lines with the exception of methionine 207, which is shown as a thick line with the sulfur atom in yellow. The bound AdoMet is also shown as a thick line. The distance of this sulfur atom to the transferable methyl group of AdoMet is 5.2, 6.7, and 7.4 Å in these three simulated conformations and is represented by black dashed lines.