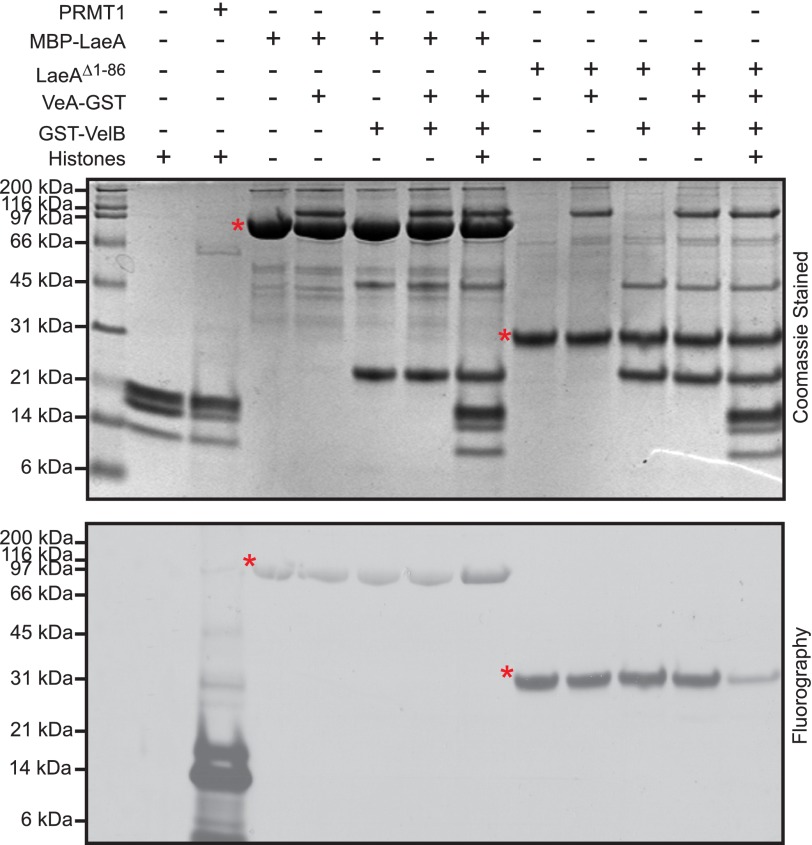
FIGURE 4.
Recombinant LaeAΔ1–86 or MBP-LaeA does not methylate purified VeA, VelB, or histone proteins. In vitro methylation reactions were prepared with 1.7 μm [3H]AdoMet and incubated for 20 h at 37 °C with methyltransferases and potential methyl-accepting substrates, including 5 μg each of recombinant human histone H2A, H2B, H3.3, and H4, 1 μg of purified PRMT1 (positive control), 27.4 μg of MBP-LaeA, ∼5 μg of VeA, ∼5 μg of VelB, and 8.8 μg of LaeAΔ1–86. The samples were separated on a 10% BisTris gel with MES running buffer and stained with Coomassie. Polypeptide molecular weight markers (Bio-Rad broad range, ∼3 μg of each protein, catalogue number 161-0317) were electrophoresed in a parallel lane and shown on the left, and include myosin (200 kDa), β-galactosidase (116 kDa), phosphorylase b (97 kDa), serum albumin (66 kDa), ovalbumin (45 kDa), carbonic anhydrase (31 kDa), trypsin inhibitor (21 kDa), lysozyme (14 kDa), and aprotinin (6 kDa). Fluorography was performed by treating the gel with EN3HANCE and exposing the dried gels to film for 5 days at −80 °C as described under “Experimental Procedures.” Red asterisks indicate the position of MBP-LaeA and LaeAΔ1–86.