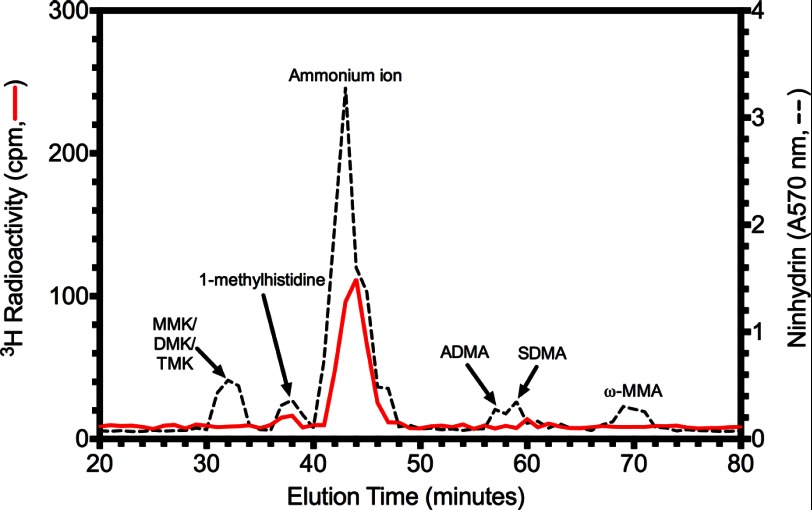
FIGURE 8.
A novel LaeA automethylated amino acid residue. 30 μg of purified LaeAΔ1–86 was incubated with 2.8 μm [3H]AdoMet for ∼20 h at 37 °C as described under “Experimental Procedures.” Reaction mixtures separated by 10% BisTris PAGE were stained, destained, and treated with EN3HANCE. After fluorography, the [3H]AdoMet-labeled LaeA protein band was excised, rehydrated with water, and acid-hydrolyzed as described under “Experimental Procedures.” After mixing with standards of methylated amino acids (1 μmol each) including 1-(π)-methylhistidine, ω-NG-monomethylarginine (ω-MMA), ω-NG,NG-dimethylarginine (ADMA), ω-NG,NG′-dimethylarginine (SDMA), ϵ-N-monomethyllysine hydrochloride (MMK), ϵ-N-dimethyllysine (DMK), and ϵ-N-trimethyllysine (TMK), high resolution cation exchange chromatography was performed as described under “Experimental Procedures.” The positions of the standard amino acids were determined by ninhydrin assay as described under “Experimental Procedures” and indicated by a dashed line. Radioactivity was determined in 200 μl of each fraction and is shown by a red solid line.