Table 1.
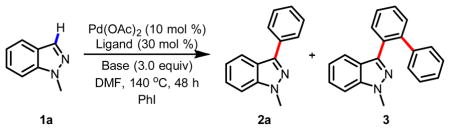
Discovery of C-3 C-H arylation of indazolea
| |||||
|---|---|---|---|---|---|
| entry | PhI (equiv) | ligand | base | 2a (%)b | 3 (%)b |
| 1 | 1.0 | Phen | Cs2CO3 | 24 | 3 |
| 2 | 2.0 | Phen | Cs2CO3 | 51 | 14 |
| 3 | 2.0 | Phen-PhMe | Cs2CO3 | 21 | 0 |
| 4 | 2.0 | Dpya | Cs2CO3 | 42 | 0 |
| 5 | 2.0 | Phen | K3PO4 | 47 | 6 |
| 6 | 2.0 | Phen | K2HPO4 | 3 | 0 |
 Phen |
 Phen-PhMe |
 Dpya |
|||
Reaction conditions: Indazole (0.25 mmol) in solvent (1 mL) at 140 °C for 48 h.
Yield was determined by 1H NMR using CH2Br2 as an internal standard.
