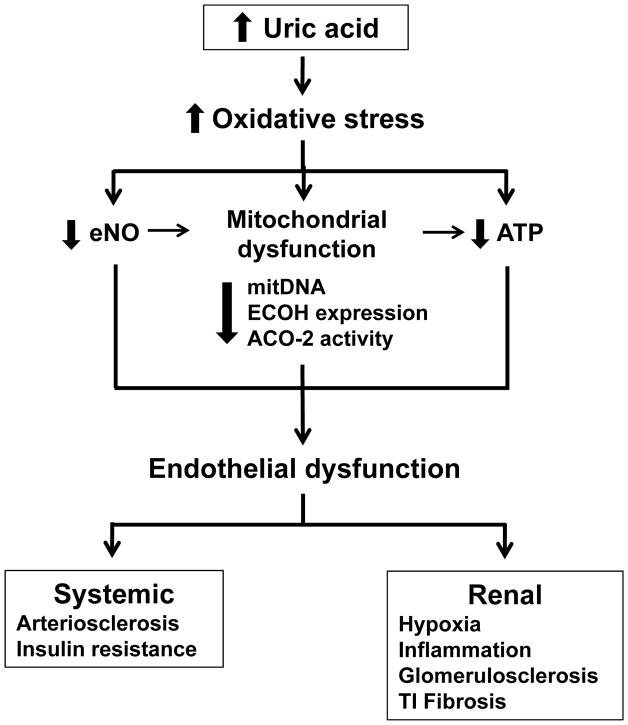
Fig. 4.
Potential mechanisms involved in uric acid-induced endothelial dysfunction and renal damage. Hyperuricaemia promotes augmented oxidative stress which in turn decreases endothelial NO bioactivity, induces mitochondrial dysfunction characterized by diminished mitochondrial mass and decreased expression or activity of the Kreb’s cycle enzymes ECoH and ACO-2 and reduced intracellular ATP. Reduced endothelial NO bioactivity may also reduce mitochondrial mass by altering biogenesis, and altered mitochondria may also contribute to reduced intracellular ATP levels. Therefore, reduced NO, mitochondrial alterations and decreased ATP results in the induction of endothelial dysfunction, which may promote arteriosclerosis and insulin resistance. In the kidney, endothelial dysfunction result in tissue hypoxia, inflammatory cell infiltration, and progressive renal disease as manifested by glomerulosclerosis and tubulointerstitial fibrosis.