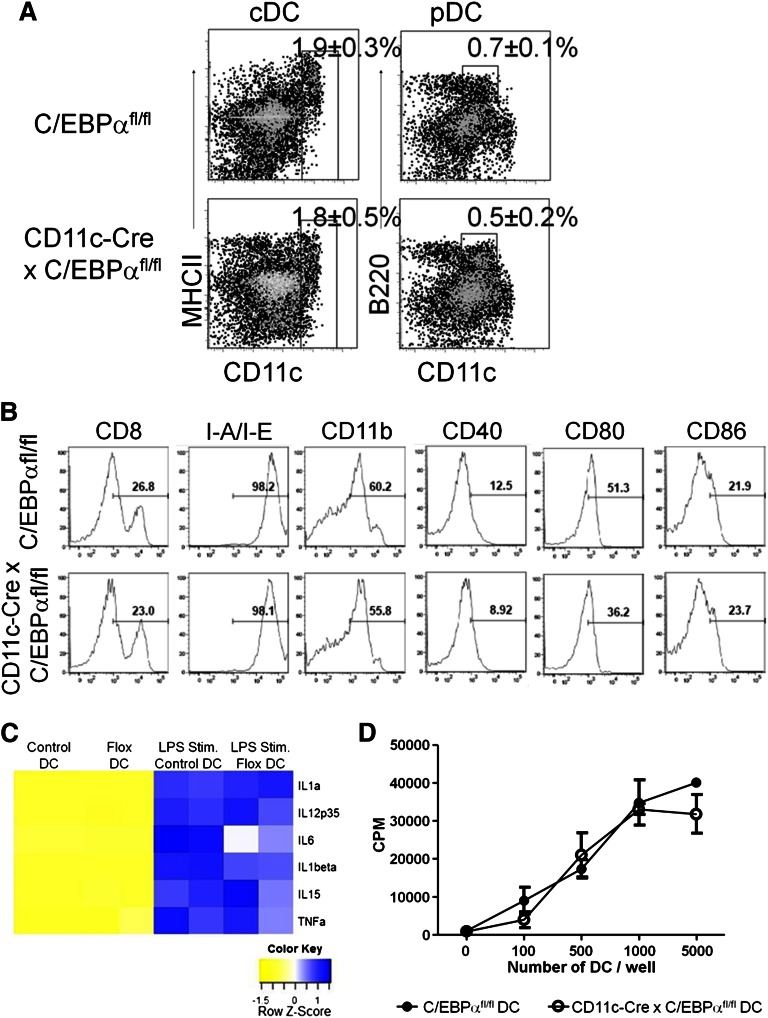
Figure 4.
Targeted C/EBPα deletion has no effect on late-stage DC development. (A) CD11c-Cre+ × C/EBPαfl/fl and C/EBPαfl/fl control mice were analyzed by flow cytometry between 8 and 12 weeks of age. Flow cytometry analysis of DC populations from the spleens of mice of the indicated genotypes are shown as dot plots. cDC are defined as CD19–, CD3–, NK–, CD11c+, and MHCII+, whereas pDC were gated as CD19–, CD3–, NK–, CD11clo, and B220+. Boxes are used to indicate the position of the DC populations in each group with the average percentage per spleen. FACS plots were taken from 3 similar experiments. (B) Histograms of flow cytometric analysis of CD11c+ splenic DC from CD11c-Cre+ C/EBPαfl/fl or C/EBPαfl/fl mice for subpopulations of DCs for CD4, CD8, CD11b, CD40, CD80, CD86, IL3Rα, and M-CSFR. Percentages are representative of similar experiments. (C) CD11c-Cre+ x C/EBPαfl/fl or C/EBPαfl/fl splenic DCs were sorted from 12-week-old mice. These cells were stimulated with LPS for 10 hours and then RT-PCR was performed for expression of cytokine transcripts. RT-PCR analysis displayed in a heatmap for transcripts from steady-state and LPS stimulated DC from both control and flox-deleted DCs. Data are shown from 2 similar experiments. (D) Control or C/EBPα-depleted DCs were sorted as CD11c+ CD19–, and CD3– into 72-hour cultures along with magnetic bead column–enriched CD3+ splenic cells to induce the proliferation of autologous T-lineage cells. The proliferative response of T-lineage cells was measured by tritiated thymidine incorporation after 16 hours. Results are representative of 2 experiments, and each value represents the mean from triplicate wells.