Abstract
Background.
Endothelial activation may contribute to development of severe disease from Plasmodium falciparum infection, but optimal markers of endothelial activation in severe malaria, the extent of endothelial activation in asymptomatic infection, and the effect of blood group O on endothelial activation have not been defined.
Methods.
Serum levels of 3 markers of endothelial activation—von Willebrand factor (VWF), soluble intercellular adhesion molecule-1 (sICAM-1), and soluble vascular cell adhesion molecule-1 (sVCAM-1)—were assessed in Ugandan children with cerebral malaria (CM) (n = 86), children with uncomplicated malaria (UM) (n = 81), and community children (CC) (n = 90).
Results.
Serum VWF, sICAM-1, and sVCAM-1 levels were all elevated in asymptomatic community children with microscopy-confirmed parasitemia when compared with children without parasitemia by microscopy or polymerase chain reaction (all, P ≤ .05). Levels of VWF, sICAM-1, and sVCAM-1 were higher in children with UM than in CC (all, P < 0.001), but only VWF levels effectively distinguished CM from UM (P < 0.001), a finding confirmed by receiver operating characteristic analyses (area under the curve = 0.67; 95% confidence interval, .58–.75). Von Willebrand factor levels were lower in children with blood group O versus non-O blood groups across the disease spectrum, but VWF levels remained higher in CM versus UM, even after controlling for blood group.
Conclusions.
Endothelial activation, as assessed by serum levels of VWF, sICAM-1, and sVCAM-1, occurs even in subclinical P. falciparum parasitemia. Von Willebrand factor levels increase with greater malaria disease severity. Blood group O is associated with lower VWF levels, but presence of blood group O alone does not explain the higher VWF levels seen in children with CM.
Cerebral malaria (CM) is a deadly disease that affects hundreds of thousands of children worldwide [1]. The pathogenesis of CM is not fully defined, but it is thought to involve parasite sequestration in postcapillary venules, leading to tissue hypoxia and ischemic damage, and immune responses to Plasmodium falciparum, notably pro-inflammatory cytokine responses [2]. Parasite sequestration is a hallmark in the brain pathology of children who die of CM (reviewed in [3]), and activation of endothelial cells by infected erythrocytes and pro-inflammatory cytokines, notably tumor necrosis factor, is thought to contribute to parasite sequestration and attraction of leukocytes and platelets to the areas of microvascular parasite sequestration [4]. However, the process of endothelial activation and the markers that best identify activation are still not clearly understood.
Studies have shown P. falciparum infection and severe malaria correlates with an increase in several markers of endothelial activation, including von Willebrand factor (VWF), soluble intercellular adhesion molecule-1 (sICAM-1), and soluble vascular cell adhesion molecule-1 (sVCAM-1) [5–11]. Intercellular adhesion molecule-1 and VCAM-1 bind infected erythrocytes, assist in leukocyte extravasation, and can induce the local release of cytokines as well as the production of tissue factor, which is the principle initiator of coagulation [12, 13]. Von Willebrand factor functions directly in primary hemostasis, mediating platelet binding and aggregation to components of the extracellular matrix, and indirectly in secondary hemostasis as a carrier protein for factor VIII [14].
In malaria, blood group A has been associated with severe disease and blood group O with protection from severe disease [15, 16]. A number of studies report plasma VWF levels 25%–30% lower in O group individuals than non-O group individuals [17, 18]. We hypothesized that the protection from severe disease afforded by blood group O may be related to decreased endothelial activation in those with this blood group. To test this hypothesis and to assess which marker of endothelial activation best predicts severe disease with malaria, we compared serum levels of VWF, sICAM-1, and sVCAM-1, and O and non-O blood group phenotypes in children with CM, children with uncomplicated malaria (UM), and community children (CC).
METHODS
Study Population and Plasmodium falciparum Detection
The study was conducted at Mulago Hospital, Kampala, Uganda. Children aged 4–12 years with CM (n = 88) or UM (n = 81) or asymptomatic CC (n = 100) were recruited as part of 2 studies assessing the complications of CM. Complete details of the study cohorts and the control samples were previously reported [19]. Written informed consent was obtained from the parents or guardians of study participants. The Institutional review boards for human studies at Makerere University Faculty of Medicine, University Hospitals of Cleveland, Case Western Reserve University, University of Minnesota, and Indiana Wesleyan University granted ethical approval for the study. In addition to microscopy on blood smear, nearly all study participants were screened by polymerase chain reaction (PCR) for P. falciparum according to methods previously published [20]. Asymptomatic parasitemia analyses were performed only on community children in whom both microscopy and PCR testing were done (n = 67).
Immunoassays
Serum samples were available for testing from 86 children with CM, 81 children with UM, and 90 CC, but volume requirements limited the number of samples tested for each assay. Serum samples were kept frozen at −70°C until testing. The relative concentration of VWF (VWF:antigen) is expressed as a percent of normal and was determined using a commercial enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions (Corgenix, Inc). To keep measurements within the range of the standard curve, some samples were additionally diluted either 1:3 or 1:5 from the protocol recommendations in the sample diluent provided in the kit. Serum concentrations of sVCAM-1 and sICAM-1 were measured with commercial ELISAs according to the manufacturer's instructions (R&D Systems) diluted 1:20 in the diluent provided in the kit. At least 10 samples were tested in duplicate in separate assays to assess reliability of sample testing, and the mean differences in replicate values of all assays were <10%.
ABO Blood Group Genotyping
Genomic DNA samples were purified from filter paper blood spots from 77 children with CM, 80 children with UM, and 83 CC using a QIAamp DNA Blood Kit (QIAGEN). Eluted concentrations ranged 1–50 ng/μL. ABO blood group genotyping was performed similar to a method published by Olsson and Chester [21]. Two separate PCR reactions were used to amplify 2 regions of the ABO locus, and enzymatic digestions of the resulting products were used to discriminate between O and non-O blood groups. Although there are limitations in detecting subgroup alleles, hybrid genes, and null genes with this method, the common genotypes with alleles A1, A2, B, O1, and O2 are correctly identified [22]. Two primer pairs were used in the reactions: ABO114f: 5'- GCATTTGCCTCTGGTTGGTT-3' with ABO421r: 5'-GAGACGCAGCCTCTGGAGAAG-3'; and gp101f: 5'-CCGTCCGCCTGCCTTGCAGAT-3' with gp71r: 5'-CTAGGCTTCAGTTACTCACAA-3'. All PCR reactions utilized iTaq DNA polymerase and the included kit components (Bio-Rad) according to the manufacturer's instructions with 10 µL reaction volumes containing 1 µL of gDNA and primer concentrations of 250 nM in a Tetrad 2 Thermal Cycler (Bio-Rad). Primers ABO114f and ABO421r amplify the ABO gene exon 6 and regions of both the upstream and downstream introns by denaturation at 95oC for 5 minutes followed by 40 cycles of 95oC for 30 seconds (ramping rate of 2.5oC/s), 64oC for 2 minutes (ramping rate of 0.5oC/s), and 72oC for 1 minute (ramping rate of 2.5oC/s), with a final 72oC for 10 minutes. The resulting 534/535 base-pair product was digested with 5U of KpnI (New England Biolabs) for 2 hours at 37oC and then analyzed in 1% Tris/Borate/ethylenediaminetetraacetic acid agarose gels containing 0.5 µg/mL of ethidium bromide with a FOTO/Analyst Investigator/Eclipse System (FOTODYNE Inc). Primers gp101f and gp71r were used to amplify the majority of exon 7 of the ABO gene with the same cycling procedures as listed above except for an annealing temperature of 62.5oC. The resulting 835 base-pair product was digested with 5U of HpaII (New England Biolabs) for 2 hours at 37oC and analyzed in 3% sodium boric acid agarose gels [23] containing 0.5 µg/mL of ethidium bromide.
Statistical Analyses
Statistical analyses were performed with STATA SE version 10.1 (StataCorp). Von Willebrand factor, sVCAM-1, and sICAM-1 levels across disease groups and blood type groups were compared with the Wilcoxon rank sum test. ABO blood group frequencies were compared with either Fisher's exact test or Pearson's χ2 test. Significance was defined at P < .05. Receiver operating characteristic analyses were performed between groups for each endothelial marker. Multiple comparisons were adjusted for by the Bonferroni correction.
RESULTS
Study Participant Characteristics
Children with CM were more frequently male and younger and had lower hemoglobin levels, lymphocyte counts, and platelet counts than children with UM or CC (Table 1). Children with CM also had higher median parasite density than CC but not children with UM (Table 1). Sixty-seven of the 90 CC were assessed for P. falciparum infection by both microscopy and PCR. Age, sex, weight, hemoglobin level, and lymphocyte count did not differ in children by microscopy or PCR P. falciparum infection status (Table 1). However, platelet count was lower in children who were microscopy and PCR positive compared with children who were microscopy negative, PCR positive, or microscopy and PCR negative (Table 1).
Table 1.
Demographic and Hematological Characteristics of Study Participants
| Cerebral Malaria (CM) | Uncomplicated Malaria (UM) | Community Children (CC)f | PCR(+) Smear(+) | PCR(+) Smear(-) | PCR(-) Smear(-) | |
|---|---|---|---|---|---|---|
| Characteristic | (n = 86) | (n = 81) | (n = 90) | (n = 20) | (n = 32) | (n = 15) |
| Sex, % male | 59.3a | 40.7 | 48.9 | 50.0 | 43.8 | 46.7 |
| Age, years | 5.4 (4.3–7.0)ab | 6.8 (4.9–9.0) | 7.2 (6.0–9.6) | 7.4 (5.9–10.4) | 7.0 (6.1–9.7) | 7.2 (5.7–10.0) |
| Weight, kg | 16.9 (14.0–21.0)ab | 20.0 (16.0–23.8) | 21.0 (17.5–26.5) | 19.8 (16.0–29.0) | 20.0 (18.1–24.5) | 22.0 (17.5–26.0) |
| Hemoglobin, mg/dL | 7.6 (6.3–9.6)ab | 11.0 (9.7–12.3)c | 12.2 (11.0–12.7) | 12.0 (10.9–12.7) | 12.4 (11.1–13.0) | 12.2 (10.9–12.3) |
| Lymphocyte, cells/mL | 2.8 (1.8–3.7)ab | 2.0 (1.6–2.8)c | 3.7 (3.1–4.6) | 4.5 (3.1–5.2) | 3.4 (2.8–-4.5) | 3.7 (2.3–4.3) |
| Platelet, cells/mL | 83 (56–142)ab | 156 (104–207)c | 321 (267–383) | 266 (211–338)e | 336 (286–405) | 328 (295–421) |
| Parasite density | 29 760 (4000–148 680)b | 32 200 (5700–116 240)c | 4960 (460–6960)d | 5880 (460–6960) | NA | NA |
Data are median (interquartile range), unless otherwise indicated. Sex was compared by χ2 testing (sex); all other variables by or Wilcoxon rank sum testing.
Abbreviations: NA, not applicable; PCR, polymerase chain reaction.
aCM to UM, P < .05.
bCM to CC, P < .05.
cUM to CC, P < .05.
dOnly microscopy (smear) (+), asymptomatic parasitemia CC.
ePCR(+)Smear(+) to both PCR(+)Smear(-) and PCR(-)Smear(-), P < .05.
fOnly CC with both PCR and microscopy results included in asymptomatic parasitemia analyses.
Von Willebrand Factor Antigen Levels According to Disease Severity and Parasitemia
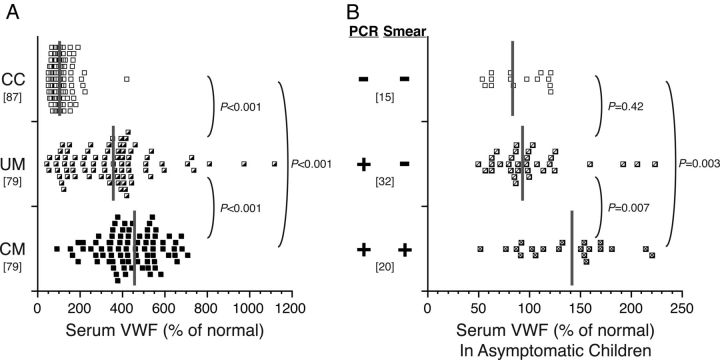
Levels of VWF (median level as percent of normal [interquartile range]) were significantly higher in children with UM compared with CC (357 [177–428] vs 102 [80–129]; P < .001) and increased further in children with CM compared with children with UM (CM, 427 [352–539]; P < .001 compared with UM) (Figure 1A). Levels of VWF correlated with parasite density in children with UM (Spearman's ρ = 0.35; P = .002) but not in children with CM (ρ = 0.19; P = .11).
Figure 1.
Serum von Willebrand factor (VWF) levels are higher in children with cerebral malaria (CM) than uncomplicated malaria (UM) and are elevated even in children with asymptomatic parasitemia. Values are reported as a relative percent (%) of normal. Gray vertical bars indicate the median, and the bracketed numbers indicate number of samples tested. A, Serum VWF levels in children with CM, children with UM, and community children (CC). B, Serum VWF levels in asymptomatic community children, according presence or absence of Plasmodium falciparum in study participants as detected by microscopy (Smear) or polymerase chain reaction (PCR).
Among asymptomatic CC, VWF levels (median [interquartile range]) were higher in children with peripheral blood parasitemia by both microscopy and PCR (141 [97–164]) than in children with parasitemia by PCR only (93 [80–116]; P = .003) or children without parasitemia by either microscopy or PCR (84 [63–119]; P = .007) (Figure 1B).
Von Willebrand Factor Antigen Levels According to ABO Blood Group and Disease Severity
There was no significant difference in the frequency of the blood group O phenotype between CC (48%) and children with UM (49% O; P = .94) or CM (44%; P = .61). The percentage with blood group O, A, B, and AB was 44%, 23%, 26%, and 7%, respectively, for children with CM, 49%, 26%, 16%, and 9%, respectively, for children with UM (P = .51 compared with CM), and 48%, 33%, 12%, and 7%, respectively, for CC (P = .14 compared with CM; P = .75 compared with UM). Blood group O was associated with lower VWF levels in all study groups (Figure 2A). Von Willebrand factor levels were higher in children with CM compared with children with UM whether the children had blood group O (CM vs UM, P = .005) or had a non-O blood group (CM vs UM, P = .009) (Figure 2A). However, VWF levels between children with CM and blood group O and children with UM and non-O blood groups did not differ significantly (P = .39) (Figure 2A), highlighting the contribution of blood group O to VWF levels and the importance of assessment of blood group when comparing VWF levels with disease severity.
Figure 2.
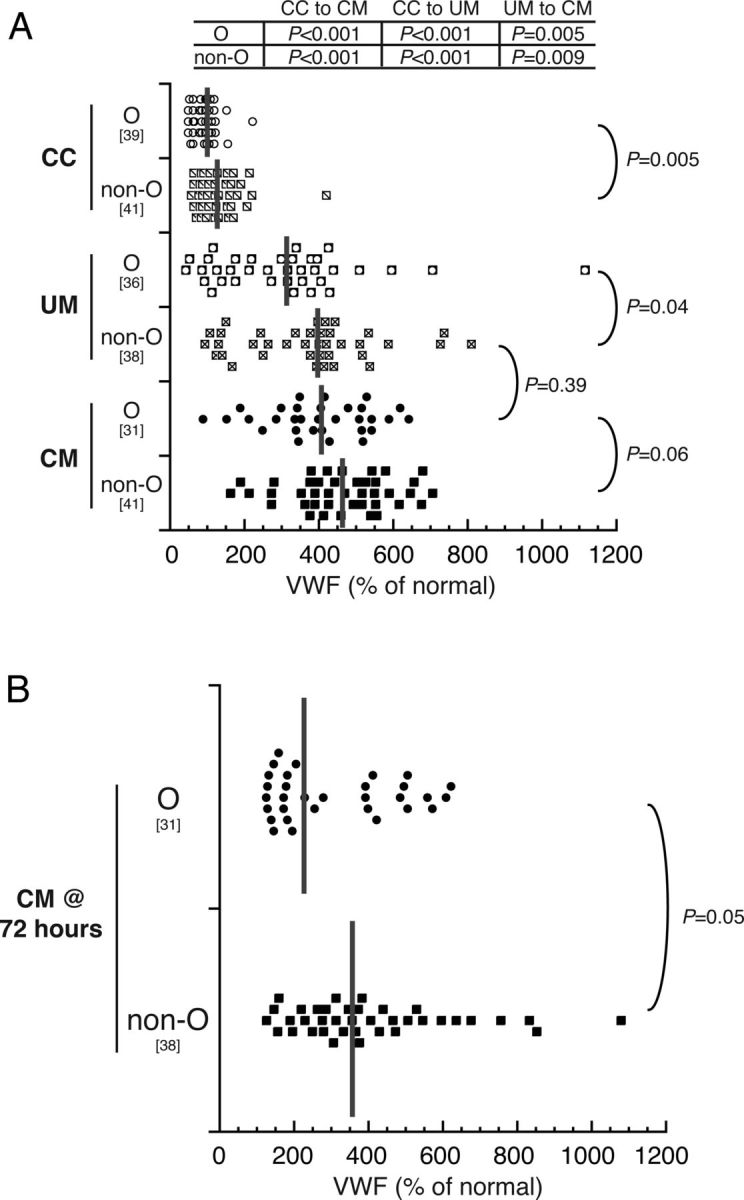
Von Willebrand factor (VWF) levels are higher in non-O blood group children than O blood group children and decrease after 72 hours. Enzyme-linked immunosorbent assay results show the distribution of VWF levels as a percent of normal. The gray horizontal bars indicate the median, and the bracketed numbers indicate number of samples tested. Group VWF levels were compared with Wilcoxon rank sum tests, and P values are reported for the different comparisons. A, Von Willebrand factor levels in O and non-O phenotypes in community children (CC), children with uncomplicated malaria (UM), and children with cerebral malaria (CM). B, Serum VWF levels 72 hours postadmission in the children with CM, O and non-O blood groups.
Von Willebrand factor levels in children with CM with O or non-O blood groups both decreased significantly at a 72-hour time point (P = .02 for O group; P = .03 for non-O groups), but levels in the non-O group remained higher than those in the O group (Figure 2B). Von Willebrand factor levels on enrollment correlated strongly with platelet count in children with UM (ρ = −0.50; P < .001) but not in children with CM (ρ = −0.12; P = .32) or asymptomatic CC (ρ = −0.11; P = .59).
Levels of sICAM-1 and sVCAM-1 According to Disease Severity, Parasitemia, and ABO Blood Group
Levels of sICAM-1 (median, ng/mL [interquartile range]) were significantly higher in children with CM (500 [359–747]) or UM (507 [315–647] than in community children (299 [186–478]; P < .001) but did not differ between children with CM and UM (P = .41) (Figure 3A). Levels of sICAM-1 were significantly higher in asymptomatic community children with parasitemia by microscopy (450 [288–553]) compared with those with parasitemia by PCR only (252 [181–455]; P = .03) but were not significantly different from aparasitemic children (296 [192–466]; P = .18) (Figure 3B) or from children with UM (P = .12) (data not shown). Levels of sICAM-1 correlated to parasite density in children with UM (ρ = 0.23; P = .04) and in microscopy-positive CC (ρ = 0.36; P = .04) but were not correlated in children with CM (ρ = 0.04; P = .77).
Figure 3.
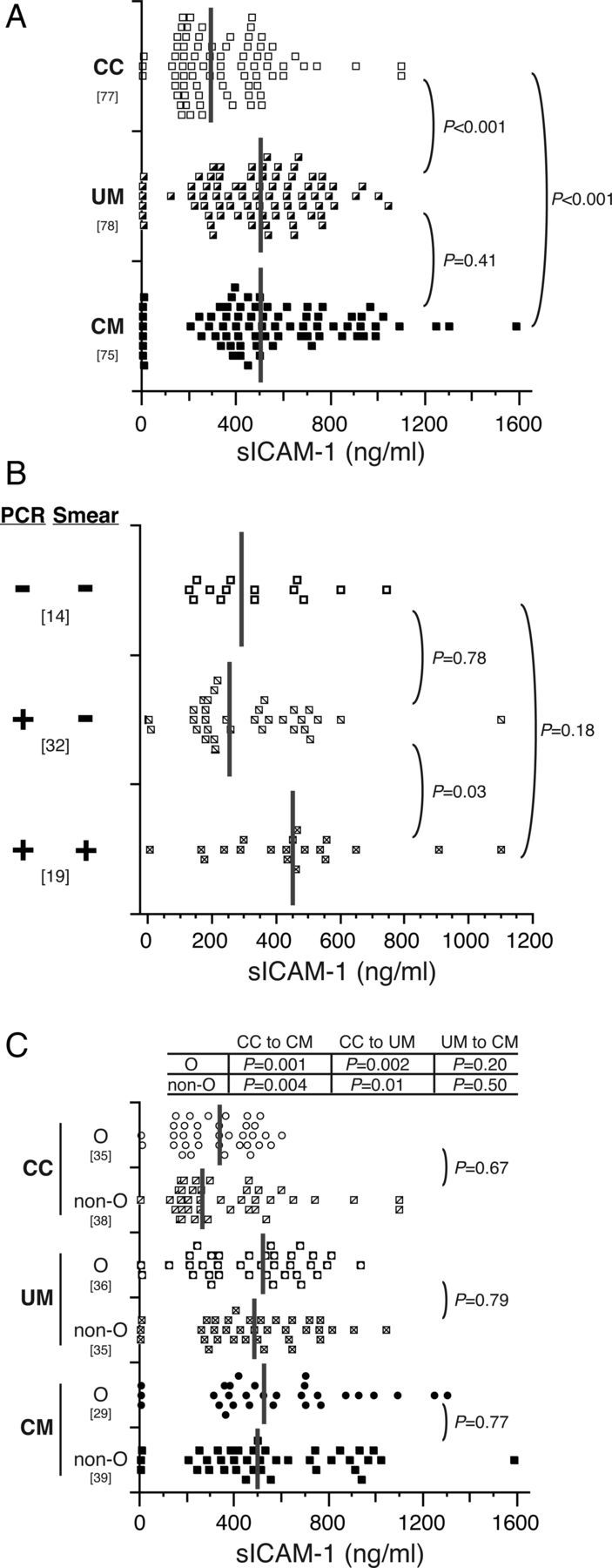
sICAM-1 levels do not differ and are not affected by ABO blood group in children with uncomplicated malaria (UM) and cerebral malaria (CM). A, Serum concentrations of sICAM-1 were determined for community children (CC) and children with UM or CM. B, Serum concentrations of sICAM-1 from CC were subgrouped according to the presence or absence of parasites as detected by polymerase chain reaction (PCR) and microscopy (Smear). Only CC with both PCR and microscopy results are shown. C, Serum concentrations of sICAM-1 were subgrouped by O and non-O blood group. Dot plots show the resulting distribution of values (ng/mL), with medians indicated on the plot with vertical gray bars, and the bracketed numbers indicate number of samples tested. Group levels were compared with Wilcoxon rank sum tests and P values are reported for the indicated group comparisons.
Levels of sICAM-1 did not differ between children with O versus non-O blood group regardless of whether the children had CM or UM or were CC (all, P > .05) (Figure 3C). No significant differences of sICAM-1 levels were seen between children with any individual blood group (data not shown).
Levels of sVCAM-1 were significantly higher in children with CM (3017 [2531–3456]) or UM (2781 [2163–3294]) than in CC (1325 [775–1877]; P < .001) and were modestly elevated in children with CM compared with children with UM (P = .06) (Figure 4A). Levels of sVCAM-1 were significantly higher in asymptomatic CC with parasitemia by microscopy (1739 [1423–2187]) compared with those with parasitemia by PCR only (1063 [636–1438]; P = .001) but were not different to aparasitemic children (1187 [707–2351]; P = 0.08) (Figure 4B). However, the sVCAM-1 levels of the microscopy-positive CC were significantly lower than those in children with UM (P < .001) (data not shown). Levels of sVCAM-1 correlated to parasite density in children with CM (ρ = 0.30; P = .007) or UM (ρ = 0.43; P < .001) and in microscopy-positive CC (ρ = 0.53; P = .001).
Figure 4.
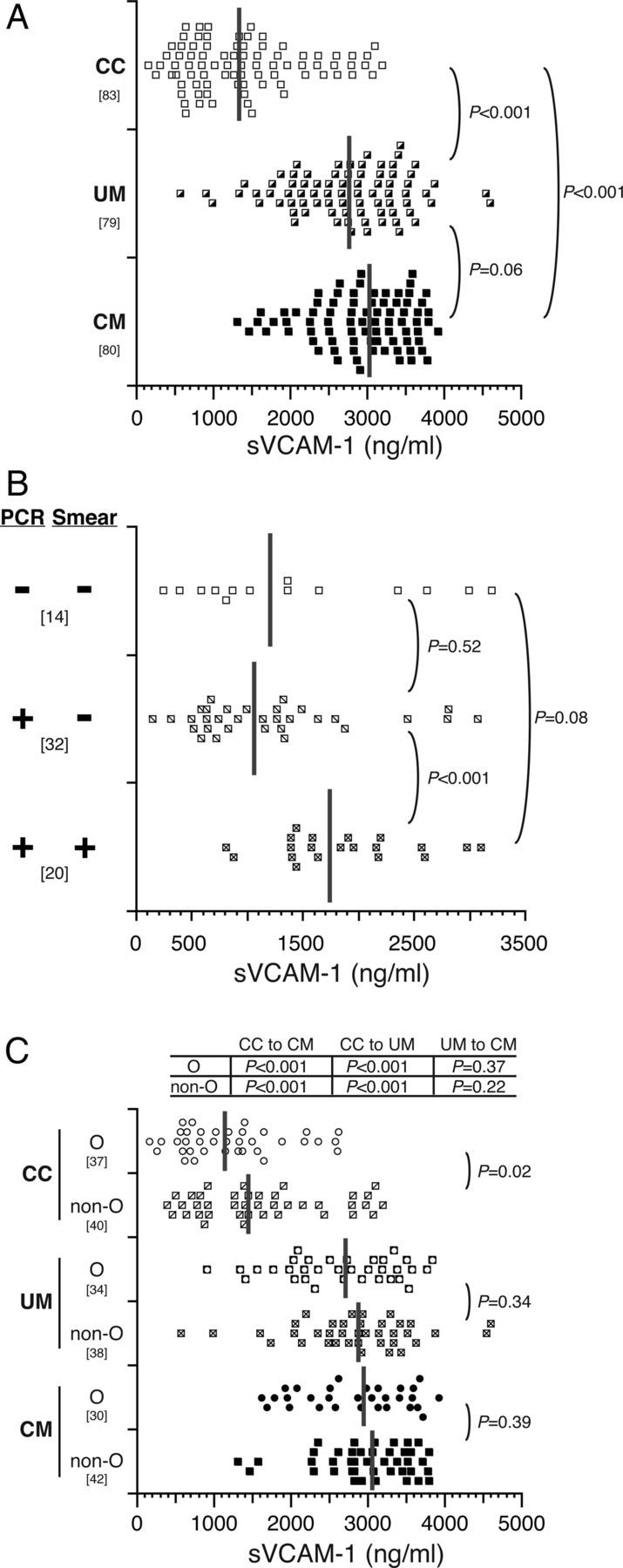
sVCAM-1 levels do not differ and are not affected by ABO blood group in children with uncomplicated and cerebral malaria. A, Serum concentrations of sVCAM-1 were determined for community children (CC) and children with uncomplicated malaria (UM) or cerebral malaria (CM). B, Serum concentrations of sVCAM-1 from CC were subgrouped according to the presence or absence of parasites as detected by polymerase chain reaction (PCR) and microscopy (Smear). Only CC with both PCR and microscopy results are shown. C, Serum concentrations of sVCAM-1 were subgrouped by O and non-O blood group. Dot plots show the resulting distribution of values (ng/mL), with medians indicated on the plot with vertical gray bars, and the bracketed numbers indicate number of samples tested. Group levels were compared with Wilcoxon rank sum tests and P values are reported for the indicated group comparisons.
Soluble vascular cell adhesion molecule-1 levels did not differ by O versus non-O blood group in children with CM or UM but were higher in non-O compared with O blood group CC (P = .02) (Figure 4C). As with sICAM-1, levels of sVCAM-1 in children with UM and CM did not differ significantly according to specific blood group (data not shown). There were no correlations of sICAM-1 levels with platelet counts in any group, but sVCAM-1 levels were correlated with platelet counts in children with CM (ρ = −0.37; P = .001) or UM (ρ = −0.44; P < .001).
Prediction of Disease Severity and Mortality by Von Willebrand Factor, sICAM-1, and sVCAM-1
Receiver operating characteristic analysis was performed for VWF, sICAM-1, and sVCAM-1. The area under the curve (AUC) for a marker that perfectly differentiates CM from UM is 1.0, whereas an AUC value of 0.5 reflects a lack of discrimination between CM and UM. Von Willebrand factor predicted disease severity better than sICAM-1 or sVCAM-1 (AUC [95% confidence interval], VWF, 0.67 [.58–.75]; sICAM-1, 0.54 [.45–.63]; sVCAM-1, 0.59 [.50–.68]). Von Willebrand factor also discriminated between parasitemic versus aparasitemic CC better than sICAM-1 or sVCAM-1 (VWF, 0.66 [.51–.81); sICAM-1, 0.54 [.36–.71]; sVCAM-1, 0.53 [.34–.73]). Finally, receiver operating characteristic analyses taking into account the O and non-O phenotypes as they related to disease severity showed very similar results (data not shown).
Von Willebrand factor, sICAM-1 and sVCAM-1 levels (median [interquartile range]) did not differ significantly in the 4 children who died [19] versus the children who survived (VWF, 566 [462–636] vs 424 [347–529], P = .09; sICAM-1, 209 [7–580] vs 500 [362–747], P = .19; sVCAM-1, 3400 [3342–3487] vs 2952 [2511–3456], P = .10).
DISCUSSION
In this study, we document that serum levels of VWF distinguish between asymptomatic children with peripheral blood parasitemia by microscopy compared with asymptomatic children without peripheral blood parasitemia by microscopy, and serum levels of VWF also distinguish UM from CM. Although levels of VWF were lower in children with blood group O, blood group alone did not account for the differences seen in VWF levels between children with UM versus CM. Serum levels of 2 other markers of endothelial activation, sICAM-1 and sVCAM-1, were also higher in asymptomatic children with peripheral blood parasitemia compared with asymptomatic children without parasitemia but did not distinguish UM from CM. Our findings provide evidence that endothelial activation occurs in asymptomatic children with peripheral P. falciparum parasitemia, at least at the level required for detection by microscopy, and suggest that VWF may be a particularly useful marker of endothelial activation in malaria. Our findings also provide impetus for further assessment of the role of VWF in the pathogenesis of severe malaria and UM.
Among the most interesting study findings was that endothelial activation as measured by all 3 markers was seen even in children with asymptomatic parasitemia, if parasitemia reached the level detectable by microscopy. In a prior study in which malaria-naive humans were infected with P. falciparum, endothelial activation as assessed by elevated VWF levels occurred when parasitemia was detected by PCR, prior to detection by microscopy. In the present study, children in a malaria-endemic area demonstrated elevation of VWF levels only when parasitemia reached levels detected by microscopy. The differences may relate to a difference in endothelial responses to P. falciparum–infected erythrocytes in a malaria-naive host versus a previously malaria-exposed host [6]. Chronic endothelial activation in other diseases (eg, sickle cell anemia [24], systemic lupus erythematosus [25]) appears to lead to adverse outcomes, including endothelial dysfunction, atherosclerosis, vasoocclusion, and multiple organ damage. Our study findings have shown cognitive impairment in children with CM [26], but other studies have shown developmental consequences even in children with repeated episodes of UM [27]. Children in highly malaria-endemic areas frequently have chronic asymptomatic parasitemia by microscopy [28, 29]. If endothelial activation affects brain vascular tone or causes other pathophysiology that leads to neuronal activation or damage, then the chronic endothelial activation in children with chronic subpatent parasitemia could adversely affect neurodevelopment. Chronic endothelial activation could also lead to other complications such as ischemia and reperfusion injuries or apoptosis of endothelial cells leading to a breakdown of the microvascular barrier [30]. The finding of endothelial activation in asymptomatic parasitemia suggests that chronic subclinical parasitemia could have adverse long-term clinical consequences [31]. In addition, the finding of a lower platelet count in children with asymptomatic parasitemia at the level detectable by microscopy further supports the presence of a subclinical hematological response to P. falciparum in these children. Future studies should assess endothelial activation in children with repeated asymptomatic parasitemia and whether chronic neurodevelopmental or cardiac disease is more frequent in these children on long-term follow-up. Any association of chronic endothelial activation with chronic disease would provide a further argument for malaria elimination as opposed to control.
Von Willebrand factor, but not ICAM-1 or VCAM-1, discriminated between those with severe versus mild disease, suggesting that VWF not only reflects endothelial activation but may be involved in disease pathogenesis. Our study findings confirm those of recently published studies in which VWF and VWF propeptide levels were higher in children with CM than in children with mild malaria [8, 9]. The present study adds to these studies by assessing levels of additional markers of endothelial activation (ICAM-1, VCAM-1), investigating levels in asymptomatic parasitemia, and assessing the contribution of blood group to VWF levels across the disease spectrum. Whereas ICAM-1 and VCAM-1 can bind infected erythrocytes (IEs) to endothelial cells, providing a mechanism for the sequestration of IEs, VWF is thought to mediate IE adhesion to endothelial cells via platelets and CD36 [32, 33]. Platelets may play a role in killing asexual blood stages of P. falciparum [34], so increases in VWF might lead to an increase in disease severity because of the role of VWF in binding platelets and inhibiting their protective effect, in addition to providing another mechanism for the sequestration of IEs [34]. In support of this, we found that levels of VWF were negatively correlated with platelet counts (ρ = −0.50; P < .001) and parasitemia in children with UM. Von Willebrand factor activity might also contribute to the thrombocytopenia seen in severe malaria, though recent studies have suggested that thrombocytopenia is not a result of consumptive coagulopathy [35].
Other studies have shown decreased plasma concentrations and activity of the VWF processing enzyme ADAMTS13 in severe P. falciparum malaria [36–38]. ADAMTS13 specifically cleaves ultralarge (UL)–VWF in conditions of fluid stress into less active/prothrombotic, lower molecular weight multimers [39]. It would be of interest to determine if the children with asymptomatic parasitemia had a decrease in ADAMTS13 activity and/or an increase in UL-VWF multimers. Such investigations would help to elucidate the effect of a low-level parasitemia on a chronic activation of the endothelium.
Blood group differences explained some, but not all, of the differences in VWF levels between children with UM and severe malaria. Von Willebrand factor contains ABO N-linked and O-linked glycosylation sites, and VWF proteolysis by ADAMTS13 differs between blood groups in the processing of UL-VWF into dimeric proteolytic fragments [40–42]. Because O group individuals have no A or B carbohydrates, VWF is processed more readily, leading to lower levels of UL-VWF. It has also been shown that ADAMTS13 functional activity on VWF can be influenced by ABO blood group via sialylation [41, 43]. These findings suggest mechanisms by which the previously documented association of blood group O with protection from severe malaria [15, 44] may be mediated in part by alterations in VWF processing and level. Our study demonstrates that assessment of ABO blood group is critical when analyzing VWF levels in populations with severe malaria. Future studies should further assess the mechanisms by which ABO group may alter VWF level and function in severe malaria.
In summary, this study provides strong evidence that endothelial activation occurs even in children with asymptomatic P. falciparum parasitemia and that VWF levels distinguish CM from UM. Although VWF levels were lower in children with blood group O compared with children with non-O blood groups, blood group alone did not account for the differences seen in VWF levels between children with UM versus CM. Future studies should assess whether VWF interactions with blood group and platelets play a role in malaria pathogenesis and whether chronic endothelial activation in children with asymptomatic parasitemia is associated with long-term developmental or other consequences.
Acknowledgments
We thank the study participants and their guardians for their participation in the study. We also thank the study team, including medical officers, nurses, and data entry personnel, Tracy Bergmann for statistical assistance, Gregory Vercelotti for manuscript discussions, and Allison Gurney for laboratory assistance.
Financial support. This work was supported by grants from the National Institutes of Health Fogarty International Center (R21 TW006794 to C. C. J.); the National Institute of Neurological Disorders and Stroke at the National Institutes of Health (R01 NS055349 to C. C. J.); and the University of Minnesota Undergraduate Research Opportunities Program (to K. F. I.).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Breman JG, Alilio MS, Mills A. Conquering the intolerable burden of malaria: what's new, what's needed: a summary. Am J Trop Med Hyg. 2004;71:1–15. [PubMed] [Google Scholar]
- 2.van der Heyde HC, Nolan J, Combes V, Gramaglia I, Grau GE. A unified hypothesis for the genesis of cerebral malaria: sequestration, inflammation and hemostasis leading to microcirculatory dysfunction. Trends Parasitol. 2006;22:503–8. doi: 10.1016/j.pt.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Haldar K, Murphy SC, Milner DA, Taylor TE. Malaria: mechanisms of erythrocytic infection and pathological correlates of severe disease. Annu Rev Pathol. 2007;2:217–49. doi: 10.1146/annurev.pathol.2.010506.091913. [DOI] [PubMed] [Google Scholar]
- 4.Clark IA, Rockett KA. The cytokine theory of human cerebral malaria. Parasitol Today. 1994;10:410–2. doi: 10.1016/0169-4758(94)90237-2. [DOI] [PubMed] [Google Scholar]
- 5.Hollestelle MJ, Donkor C, Mantey EA, et al. von Willebrand factor propeptide in malaria: evidence of acute endothelial cell activation. Br J Haematol. 2006;133:562–9. doi: 10.1111/j.1365-2141.2006.06067.x. [DOI] [PubMed] [Google Scholar]
- 6.de Mast Q, Groot E, Lenting PJ, et al. Thrombocytopenia and release of activated von Willebrand factor during early Plasmodium falciparum malaria. J Infect Dis. 2007;196:622–8. doi: 10.1086/519844. [DOI] [PubMed] [Google Scholar]
- 7.Turner GD, Ly VC, Nguyen TH, et al. Systemic endothelial activation occurs in both mild and severe malaria. Correlating dermal microvascular endothelial cell phenotype and soluble cell adhesion molecules with disease severity. Am J Pathol. 1998;152:1477–87. [PMC free article] [PubMed] [Google Scholar]
- 8.Phiri HT, Bridges DJ, Glover SJ, et al. Elevated plasma von Willebrand factor and propeptide levels in Malawian children with malaria. PLoS One. 2011;6:e25626. doi: 10.1371/journal.pone.0025626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erdman LK, Dhabangi A, Musoke C, et al. Combinations of host biomarkers predict mortality among Ugandan children with severe malaria: a retrospective case-control study. PLoS One. 2011;6:e17440. doi: 10.1371/journal.pone.0017440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conroy AL, Phiri H, Hawkes M, et al. Endothelium-based biomarkers are associated with cerebral malaria in Malawian children: a retrospective case-control study. PLoS One. 2010;5:e15291. doi: 10.1371/journal.pone.0015291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conroy AL, Lafferty EI, Lovegrove FE, et al. Whole blood angiopoietin-1 and -2 levels discriminate cerebral and severe (non-cerebral) malaria from uncomplicated malaria. Malar J. 2009;8:295. doi: 10.1186/1475-2875-8-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jenkins N, Wu Y, Chakravorty S, et al. Plasmodium falciparum intercellular adhesion molecule-1–based cytoadherence-related signaling in human endothelial cells. J Infect Dis. 2007;196:321–7. doi: 10.1086/518795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Francischetti IM, Seydel KB, Monteiro RQ. Blood coagulation, inflammation, and malaria. Microcirculation. 2008;15:81–107. doi: 10.1080/10739680701451516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blann AD. Plasma von Willebrand factor, thrombosis, and the endothelium: the first 30 years. Thromb Haemost. 2006;95:49–55. [PubMed] [Google Scholar]
- 15.Rowe JA, Handel IG, Thera MA, et al. Blood group O protects against severe Plasmodium falciparum malaria through the mechanism of reduced rosetting. Proc Natl Acad Sci U S A. 2007;104:17471–6. doi: 10.1073/pnas.0705390104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fry AE, Griffiths MJ, Auburn S, et al. Common variation in the ABO glycosyltransferase is associated with susceptibility to severe Plasmodium falciparum malaria. Hum Mol Genet. 2008;17:567–76. doi: 10.1093/hmg/ddm331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jenkins PV, O'Donnell JS. ABO blood group determines plasma von Willebrand factor levels: a biologic function after all? Transfusion. 2006;46:1836–44. doi: 10.1111/j.1537-2995.2006.00975.x. [DOI] [PubMed] [Google Scholar]
- 18.Klarmann D, Eggert C, Geisen C, et al. Association of ABO(H) and I blood group system development with von Willebrand factor and factor VIII plasma levels in children and adolescents. Transfusion. 2010;50:1571–80. doi: 10.1111/j.1537-2995.2010.02604.x. [DOI] [PubMed] [Google Scholar]
- 19.John CC, Opika-Opoka R, Byarugaba J, Idro R, Boivin MJ. Low levels of RANTES are associated with mortality in children with cerebral malaria. J Infect Dis. 2006;194:837–45. doi: 10.1086/506623. [DOI] [PubMed] [Google Scholar]
- 20.Menge DM, Ernst KC, Vulule JM, et al. Microscopy underestimates the frequency of Plasmodium falciparum infection in symptomatic individuals in a low transmission highland area. Am J Trop Med Hyg. 2008;79:173–7. [PMC free article] [PubMed] [Google Scholar]
- 21.Olsson ML, Chester MA. A rapid and simple ABO genotype screening method using a novel B/O2 versus A/O2 discriminating nucleotide substitution at the ABO locus. Vox Sang. 1995;69:242–7. doi: 10.1111/j.1423-0410.1995.tb02602.x. [DOI] [PubMed] [Google Scholar]
- 22.Hosseini-Maaf B, Hellberg A, Chester MA, Olsson ML. An extensive polymerase chain reaction-allele-specific polymorphism strategy for clinical ABO blood group genotyping that avoids potential errors caused by null, subgroup, and hybrid alleles. Transfusion. 2007;47:2110–25. doi: 10.1111/j.1537-2995.2007.01436.x. [DOI] [PubMed] [Google Scholar]
- 23.Brody JR, Kern SE. Sodium boric acid: a Tris-free, cooler conductive medium for DNA electrophoresis. Biotechniques. 2004;36:214–6. doi: 10.2144/04362BM02. [DOI] [PubMed] [Google Scholar]
- 24.Kaul DK, Liu XD, Zhang X, et al. Inhibition of sickle red cell adhesion and vasoocclusion in the microcirculation by antioxidants. Am J Physiol Heart and Circ Physiol. 2006;291:H167–75. doi: 10.1152/ajpheart.01096.2005. [DOI] [PubMed] [Google Scholar]
- 25.Narshi CB, Giles IP, Rahman A. The endothelium: an interface between autoimmunity and atherosclerosis in systemic lupus erythematosus? Lupus. 2011;20:5–13. doi: 10.1177/0961203310382429. [DOI] [PubMed] [Google Scholar]
- 26.John CC, Bangirana P, Byarugaba J, et al. Cerebral malaria in children is associated with long-term cognitive impairment. Pediatrics. 2008;122:e92–9. doi: 10.1542/peds.2007-3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clarke SE, Jukes MC, Njagi JK, et al. Effect of intermittent preventive treatment of malaria on health and education in schoolchildren: a cluster-randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:127–38. doi: 10.1016/S0140-6736(08)61034-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farnert A, Snounou G, Rooth I, Bjorkman A. Daily dynamics of Plasmodium falciparum subpopulations in asymptomatic children in a holoendemic area. Am J Trop Med Hyg. 1997;56:538–47. doi: 10.4269/ajtmh.1997.56.538. [DOI] [PubMed] [Google Scholar]
- 29.Ofulla AV, Moormann AM, Embury PE, et al. Age-related differences in the detection of Plasmodium falciparum infection by PCR and microscopy, in an area of Kenya with holo-endemic malaria Ann Trop Med Parasitol. 2005;99:431–5. doi: 10.1179/136485905X36316. [DOI] [PubMed] [Google Scholar]
- 30.Lee WL, Liles WC. Endothelial activation, dysfunction and permeability during severe infections. Curr Opinion Hematol. 2011;18:191–6. doi: 10.1097/MOH.0b013e328345a3d1. [DOI] [PubMed] [Google Scholar]
- 31.Clark TD, Njama-Meya D, Nzarubara B, et al. Incidence of malaria and efficacy of combination antimalarial therapies over 4 years in an urban cohort of Ugandan children. PLoS One. 2010;5:e11759. doi: 10.1371/journal.pone.0011759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Newbold C, Warn P, Black G, et al. Receptor-specific adhesion and clinical disease in Plasmodium falciparum. Am J Trop Med Hyg. 1997;57:389–98. doi: 10.4269/ajtmh.1997.57.389. [DOI] [PubMed] [Google Scholar]
- 33.Bridges DJ, Bunn J, van Mourik JA, et al. Rapid activation of endothelial cells enables Plasmodium falciparum adhesion to platelet-decorated von Willebrand factor strings. Blood. 2010;115:1472–4. doi: 10.1182/blood-2009-07-235150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McMorran BJ, Marshall VM, de Graaf C, et al. Platelets kill intraerythrocytic malarial parasites and mediate survival to infection. Science. 2009;323:797–800. doi: 10.1126/science.1166296. [DOI] [PubMed] [Google Scholar]
- 35.de Mast Q, de Groot PG, van Heerde WL, et al. Thrombocytopenia in early malaria is associated with GP1b shedding in absence of systemic platelet activation and consumptive coagulopathy. Br J Haematol. 2010;151:495–503. doi: 10.1111/j.1365-2141.2010.08399.x. [DOI] [PubMed] [Google Scholar]
- 36.de Mast Q, Groot E, Asih PB, et al. ADAMTS13 deficiency with elevated levels of ultra-large and active von Willebrand factor in P. falciparum and P. vivax malaria. Am J Trop Med Hyg. 2009;80:492–8. [PubMed] [Google Scholar]
- 37.Larkin D, de Laat B, Jenkins PV, et al. Severe Plasmodium falciparum malaria is associated with circulating ultra-large von Willebrand multimers and ADAMTS13 inhibition. PLoS Pathog. 2009;5:e1000349. doi: 10.1371/journal.ppat.1000349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lowenberg EC, Charunwatthana P, Cohen S, et al. Severe malaria is associated with a deficiency of von Willebrand factor cleaving protease, ADAMTS13. Thromb Haemost. 2010;103:181–7. doi: 10.1160/TH09-04-0223. [DOI] [PubMed] [Google Scholar]
- 39.Chauhan AK, Kisucka J, Brill A, et al. ADAMTS13: a new link between thrombosis and inflammation. J Exp Med. 2008;205:2065–74. doi: 10.1084/jem.20080130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bowen DJ. An influence of ABO blood group on the rate of proteolysis of von Willebrand factor by ADAMTS13. tJ Thromb Haemost. 2003;1:33–40. doi: 10.1046/j.1538-7836.2003.00007.x. [DOI] [PubMed] [Google Scholar]
- 41.McKinnon TA, Chion AC, Millington AJ, Lane DA, Laffan MA. N-linked glycosylation of VWF modulates its interaction with ADAMTS13. Blood. 2008;111:3042–9. doi: 10.1182/blood-2007-06-095042. [DOI] [PubMed] [Google Scholar]
- 42.Morelli VM, de Visser MC, van Tilburg NH, et al. ABO blood group genotypes, plasma von Willebrand factor levels and loading of von Willebrand factor with A and B antigens. Thromb Haemost. 2007;97:534–41. [PubMed] [Google Scholar]
- 43.McGrath RT, McKinnon TA, Byrne B, O'Kennedy R, Terraube V, et al. Expression of terminal alpha2-6-linked sialic acid on von Willebrand factor specifically enhances proteolysis by ADAMTS13. Blood. 2010;115:2666–73. doi: 10.1182/blood-2009-09-241547. [DOI] [PubMed] [Google Scholar]
- 44.Pathirana SL, Alles HK, Bandara S, et al. ABO-blood-group types and protection against severe Plasmodium falciparum malaria. Ann Trop Med Parasitol. 2005;99:119–24. doi: 10.1179/136485905X19946. [DOI] [PubMed] [Google Scholar]