Abstract
Background. Herpes simplex virus resistance to acyclovir is well described in immune-compromised patients. Management of prolonged infection and recurrences in such patients may be problematic.
Methods. A patient with neuroblastoma developed likely primary herpes gingivostomatitis shortly after starting a course of chemotherapy, with spread to the eye during treatment with acyclovir. Viral isolates were serially obtained from separate sites after treatment was begun and tested for susceptibility to acyclovir and foscarnet by plaque reduction and plating efficiency assays. The thymidine kinase and DNA polymerase genes from each isolate were sequenced.
Results. Initial isolates from a throat swab, an oral lesion, and conjunctiva were resistant to acyclovir within 13 days of treatment. Subsequent isolates while on foscarnet were initially acyclovir-susceptible, but reactivation of an acyclovir-resistant isolate was subsequently documented while on acyclovir suppression. Genotypic analysis identified a previously unreported UL23 mutation in some resistant isolates. None of the amino acid changes identified in UL30 were associated with resistance.
Conclusions. Phenotypic and genotypic antiviral resistance of herpes simplex isolates may vary from different compartments and over time in individual immune-compromised hosts, highlighting the importance of obtaining cultures from all sites. Phenotypic resistance testing should be considered for isolates obtained from at-risk patients not responding to first-line therapy. Empiric combination treatment with multiple antivirals could be considered in some situations.
Infection with herpes simplex virus (HSV) type 1 is commonly acquired in childhood, and the most frequent clinical manifestations of primary infection are gingivostomatitis and/or pharyngitis [1]. In immune-competent patients, these infections are often clinically inapparent and generally resolve without significant sequelae, although a proportion of patients have sporadic recurrent mucocutaneous lesions. Severe or prolonged disease from HSV is of greater concern in immune-compromised populations.
Although many HSV infections do not require chemotherapeutic intervention, most infections may be adequately treated with the nucleoside analogue acyclovir. This is despite the observation that both laboratory strains and clinical isolates of HSV generally contain mixtures of acyclovir-susceptible and acyclovir-resistant virus [2]. For HSV-1, 0.03% to 0.08% of infectious particles have naturally occurring mutations conferring acyclovir resistance [3, 4]. Such mutations are thought to occur at some cost to viral fitness and pathogenicity, including a relative defect in reactivation from neuronal latency [5].
Clinically significant infections with acyclovir-resistant HSV are, with few exceptions, described in immune-compromised patients [2, 6]. Selection for naturally occurring resistant virus in the absence of an effective immune response can lead to continued replication in these patients. Although such infections are uncommon in immune-competent patients, a recent report suggests a relatively high prevalence of acyclovir-resistant isolates in patients with recurrent HSV keratitis [7].
We describe an immune-compromised pediatric patient with likely primary HSV infection found to have multiple positive cultures from different sites. We analyze the antiviral susceptibility of multiple isolates from this patient separated both by anatomic site and by time and describe variable acyclovir susceptibility from these isolates. Our study highlights the importance of determining phenotypic antiviral resistance patterns in all isolates recovered from at-risk patients who appear to be failing effective antiviral therapy and suggests that in certain clinical situations combination antiviral therapy may be warranted.
Methods
Case Report
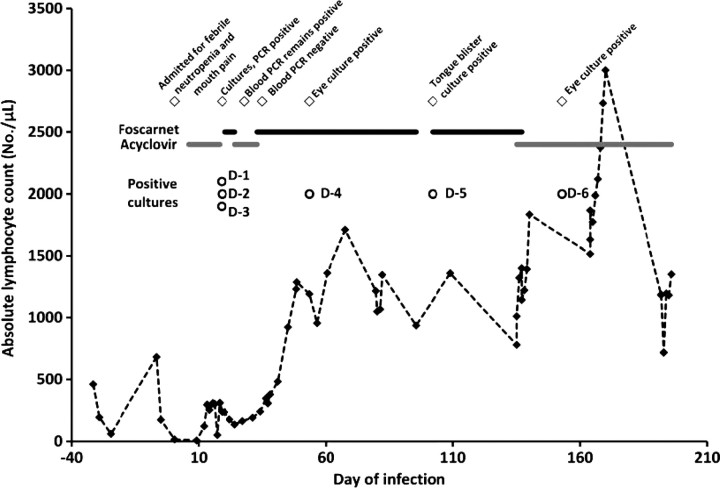
A 22-month-old patient undergoing treatment for stage 4 neuroblastoma developed mouth sores during an admission for a sixth cycle of chemotherapy. These lesions were initially attributed to mucositis, but the patient required readmission several days after discharge for febrile neutropenia and continued complaints of mouth pain (Figure 1, day 0). Direct fluorescent antigen testing (MicroTrak, Trinity Biotech) of an oral mucosal swab 5 days later was negative for HSV antigen, but empiric parenteral acyclovir was initiated (10 mg/kg/dose intravenously every 8 hours). Notably, evaluation 3 months prior in anticipation of possible autologous hematopoietic stem cell transplantation included a normal ophthalmologic examination and serologic studies that were negative for HSV-1 and HSV-2.
Figure 1.
Clinical course of patient, including relevant events (open diamonds), dates of virus isolation (open circles), antiviral courses (solid lines, with foscarnet in black and acyclovir in gray), and absolute lymphocyte count (dotted line with filled diamonds). Day 0 is the initial day of symptoms consistent with herpes simplex virus infection.
Over the next week, fever persisted, right eye injection and conjunctivitis were noted, and vesicular lesions developed on the right cheek (inferior to the lid margin) and left upper chest. Ophthalmologic examination revealed dendritic lesions of the right cornea, and topical trifluorothymidine was initiated (day 7). Cultures sent from the eye, an oral mucosal lesion, and a mouth swab were all positive for HSV-1 (day 19; these are isolates D-1 through D-3). Blood was tested by polymerase chain reaction (PCR) for HSV DNA (Viracor-IBT Laboratories) and was positive at 1100 copies/mL. The persisting symptoms and suspicion of acyclovir-resistant HSV in this patient led to a change in treatment to intravenous foscarnet (40 mg/kg/dose every 8 hours) this same day, with phenotypic resistance testing sent on the initial mouth isolate (D-1) to a commercial laboratory (Focus Diagnostics). While awaiting these results, the dendritic eye lesions resolved, and topical trifluorothymidine was tapered off by day 21.
Antiviral treatment was changed on day 24 to acyclovir at a higher dose than previously used (20 mg/kg/dose intravenously every 8 hours). Repeat blood PCR on day 28 was 500 copies/mL. Phenotypic susceptibility testing became available on day 33, and acyclovir resistance was reported for the prior mouth lesion (isolate D-1). This isolate was phenotypically susceptible to foscarnet, which was resumed, and acyclovir was discontinued. Blood PCR was negative for HSV DNA on day 35. Repeat ophthalmologic examination over this time period revealed waxing and waning severity of the right eye dendritic lesions, and trifluorothymidine was restarted on day 24 and ultimately tapered off by day 39. Fever abated, and the patient was discharged from the hospital on day 49, continuing intravenous foscarnet but on no topical ophthalmic medication.
Two days after discharge, the patient's parents noted increased right eye injection and tearing. Ophthalmologic examination on day 53 revealed recurrent dendritiform lesions of the right cornea, and topical trifluorothymidine was restarted. Culture of the eye at this time again grew HSV-1 (isolate D-4), subsequently determined to be phenotypically susceptible to acyclovir and foscarnet. Blood PCR was negative for HSV DNA. Ophthalmologic examination improved significantly after physical debridement of the cornea on day 69 of illness. By day 82 of illness, there was no corneal pathology noted, and topical treatment was discontinued. Parenteral foscarnet was discontinued on day 95.
On day 102 after the initial admission, a tongue lesion was noted at clinic follow-up. Foscarnet was restarted, and culture of the lesion was subsequently positive for HSV-1 (isolate D-5). Corneal examination remained normal. This isolate was phenotypically susceptible to acyclovir, which was resumed (10 mg/kg/dose intravenously every 8 hours). On day 153, eye symptoms, including corneal injection and periocular swelling, recurred, and HSV-1 with phenotypic resistance to acyclovir was cultured (isolate D-6). Topical trifluorothymidine was resumed, another debridement procedure was completed on day 157 of the illness, and improvement in symptoms and ophthalmologic examination was noted by day 168. Resolution of symptoms also corresponded with sustained lymphocyte numbers of above 1000 cells/μL (Figure 1). The patient has remained on suppressive oral acyclovir (20 mg/kg/dose 3 times daily) since this time with no further HSV recurrences over >4 months.
Cells and Virus Isolates
Vero cells were cultured in Dulbecco's modification of Eagle's (DME) medium plus 10% fetal bovine serum and 1% penicillin-streptomycin and were used for the propagation of the virus. Plaque titrations were performed on Vero cells by standard methods.
Serial patient HSV-1 isolates are designated D-1–D-3 (respectively isolated from an oral mucosal lesion, an eye swab, and an oropharyngeal swab on day 19), D-4 (isolated from an eye swab on day 53), D-5 (isolated from a tongue lesion on day 102), and D-6 (isolated from the eye on day 153). Initial hospital laboratory isolates were grown on MRC-5 cells; stocks from these isolates were prepared and titered on Vero cells. These limited passage stocks were used for all subsequent assays. Control strains included the laboratory strain KOS (used as a negative control for acyclovir resistance), the acyclovir-resistant strain I-309 (provided by B. Roizman, [8]), and the acyclovir-resistant, foscarnet-resistant strain 615.8 (provided by D. Coen, [9]).
Antiviral Susceptibility
Phenotypic susceptibility testing by plaque reduction was based on established methods [10, 11]. Confluent monolayers of Vero cells on 24-well plates were inoculated with 30-50 plaque-forming units of virus diluted in 250 µL phosphate-buffered saline containing 1% inactivated calf serum and 0.1% glucose. After 2 hours, the inoculum was replaced with 1 mL DMEV (DME plus 1% inactivated calf serum and 0.1% penicillin-streptomycin) containing 0.5% methylcellulose and the appropriate concentration of drug. Acyclovir (Sigma) concentrations included 220, 110, 56, 14, 3.6, 0.9, 0.4, and 0 µmol/L (50, 25, 12.5, 3.1, 0.8, 0.2, 0.1, and 0 µg/mL), and foscarnet (Sigma) concentrations included 333, 167, 83, 42, and 0 µmol/L (100, 50, 25, 12.5, and 0 µg/mL). Plaques were counted 2–3 days later, and plaque-forming units were plotted against drug concentration. The Hill slope method was used to calculate the concentration of drug that inhibits growth of virus by 50% (IC50). Each condition was run in triplicate, and positive and negative control viruses were tested along with patient isolates in each assay. Resistance is defined by IC50 ≥ 8.9 µmol/L (2 µg/mL) for acyclovir and IC50 ≥ 333 µmol/L (100 µg/mL) for foscarnet. All results reported in Tables 1–4 of this manuscript reflect testing in our laboratory, although, as noted above, the initial acyclovir resistance testing of isolate D-1 was done by a commercial laboratory.
Table 1.
Acyclovir Resistance in Serial Clinical Isolates
| Virus Isolate | Source/Day of Illness | Phenotypic Resistance to Acyclovir, IC50 (μmol/L)a | Frequency of Resistant Variants (%)b |
|---|---|---|---|
| D-1 | Mouth lesion, day 19c | 23.1 | 0.23 (23.3) |
| D-2 | Eye, day 19 | 20.9 | 0.49 (49.1) |
| D-3 | Oropharyngeal swab, day 19 | 24.0 | 0.28 (27.5) |
| D-4 | Eye, day 53 | 3.1 | 0.13 (1.32) |
| D-5 | Tongue lesion, day 102 | 3.6 | 2.0 × 10−3 (0.20) |
| D-6 | Eye, day 144 | 30.2 | 0.46 (46.4) |
| KOS | Reference strain | 8.0 | 5.9 × 10−3 (0.59) |
| I-309 | Reference strain | 77.7 | Unable to quantify |
| 615.8 | Reference strain | 16.9 | 0.44 (43.6) |
Abbreviation: IC50, median inhibitory concentration.
aDetermined by plaque reduction using Hill slope method. Breakpoint for resistance is generally IC50 ≥ 8.9 µmol/L (2 µg/mL) acyclovir [2, 10].
bDetermined by plating efficiency assay as described in “Methods.”
cTreatment with acyclovir began 6 days into the illness.
Table 2.
Thymidine Kinase Amino Acid Changes in Clinical Isolates
| Virus Strain | Thymidine Kinase Amino Acid Changesa |
||
|---|---|---|---|
| Not Associated With Resistance | Possibly Associated With Resistance | Reference | |
| D-1 | C6G | [12–17, 19, 41, 42] | |
| D-2 | N23S | [12–14, 16, 17, 19, 43] | |
| D-3 | K36E | [12–14, 16, 17, 19, 41, 43] | |
| R41H | [12–18] | ||
| A93V | |||
| A192V | [12, 14–16, 19, 41] | ||
| G251C | [12, 14–16, 19, 41, 42] | ||
| Q261R | |||
| A265T | [12–14, 17, 43] | ||
| V267L | [12, 14–16, 19, 41–43] | ||
| P268T | [12, 14–16, 19, 41–43] | ||
| D286E | [12, 14–16, 19, 41–43] | ||
| N376H | [12, 13, 15, 16, 21, 41, 42] | ||
| D-4 | C6G | [12–17, 19, 41, 42] | |
| N23S | [12–14, 16, 17, 19, 43] | ||
| D-5 | K36E | [12–14, 16, 17, 19, 41, 43] | |
| R41H | [12–18] | ||
| A192V | [12, 14–16, 19, 41] | ||
| G251C | [12, 14–16, 19, 41, 42] | ||
| Q261R | |||
| A265T | [12–14, 17, 43] | ||
| V267L | [12, 14–16, 19, 41–43] | ||
| P268T | [12, 14–16, 19, 41–43] | ||
| D286E | [12, 14–16, 19, 41–43] | ||
| N376H | [12, 13, 15, 16, 21, 41, 42] | ||
| D-6 | C6G | [12–17, 19, 41, 42] | |
| N23S | [12–14, 16, 17, 19, 43] | ||
| K36E | [12–14, 16, 17, 19, 41, 43] | ||
| R41H | [12–18] | ||
| A189V | [14, 16, 20, 21] | ||
| A192V | [12, 14–16, 19, 41] | ||
| G251C | [12, 14–16, 19, 41, 42] | ||
| Q261R | |||
| A265T | [12–14, 17, 43] | ||
| V267L | [12, 14–16, 19, 41–43] | ||
| P268T | [12, 14–16, 19, 41–43] | ||
| D286E | [12, 14–16, 19, 41–43] | ||
| N376H | [12, 13, 15, 16, 21, 41, 42] | ||
aBased on UL23 gene sequence, relative to herpes simplex virus type 1 strain 17.
Table 3.
Foscarnet Resistance in Serial Clinical Isolates
| Virus Isolate | Source/Day of Illness | Phenotypic Resistance to Foscarnet, IC50 (μmol/L)a | Frequency of Resistant Variants (%)b |
|---|---|---|---|
| D-1 | Mouth lesion, day 19c | 63 | 9.5 × 10−4 (0.095) |
| D-2 | Eye, day 19 | 123 | 3.8 × 10−3 (0.377) |
| D-3 | Oropharyngeal swab, day 19 | 93 | 7.4 × 10−4 (0.074) |
| D-4 | Eye, day 53 | 123 | 4.3 × 10−4 (0.043) |
| D-5 | Tongue lesion, day 102 | 153 | 1.1 × 10−4 (0.011) |
| D-6 | Eye, day 144 | 83 | 8.2 × 10−5 (0.008) |
| KOS | Reference strain | 137 | 8.6 × 10−3 (0.86) |
| I-309 | Reference strain | 97 | 3.1 × 10−5 (0.003) |
| 615.8 | Reference strain | >667 | 0.54 (53.9) |
Abbreviation: IC50, median inhibitory concentration.
aDetermined by plaque reduction using Hill slope method. Breakpoint for resistance is generally IC50 ≥ 333 µmol/L (100 µg/mL) foscarnet [10].
bDetermined by plating efficiency assay as described in “Methods.”
cInitial treatment with foscarnet began 19 days into the illness.
Table 4.
DNA Polymerase Amino Acid Changes in Clinical Isolates
| Virus Strain | DNA Polymerase Amino Acid Changesa |
||
|---|---|---|---|
| Not Associated With Resistance | Possibly Associated With Resistance | Reference | |
| Strains D-1 through | S33G | [12–14] | |
| D-6 had identical | A330R | [12–14] | |
| UL30 sequences | V905M | [12–14, 41] | |
| P1199Lb | |||
| T1208A | [12–14] | ||
aBased on UL30 gene sequence, relative to herpes simplex virus type 1 strain 17.
bP1199L is not previously reported, but P1199Q has been noted to be a naturally occurring amino acid change not associated with resistance [12].
A plating efficiency assay was used to determine the approximate percentage of each patient isolate resistant to a given drug [3]. Vero cells were grown to confluency on 6-well plates, and serial 10-fold dilutions of virus were inoculated onto cells. After 2 hours, the inoculum was replaced with DMEV containing 0.5% methylcellulose and either no drug, 22 µmol/L (5 µg/mL) acyclovir, or 667 µmol/L (200 µg/mL) foscarnet. Each condition was done in duplicate wells. Plaques were counted 2–3 days later, and frequency of resistant variants was calculated as (virus titer in presence of drug)/(virus titer in absence of drug). The percentage of resistant variants is 100 × (frequency of resistant variants).
DNA Sequencing
Viral DNA was isolated from working stocks according to manufacturer's instructions (Roche HighPure viral nucleic acid kit). Overlapping primer sets spanning the HSV-1 UL23 and UL30 genes (encoding viral thymidine kinase and DNA polymerase, respectively) were used to amplify these genes in each isolate (see Supplementary Material for primer sequences). Polymerase chain reaction products were sequenced bidirectionally and compared to the reference sequence from HSV-1 strain 17 (GenBank accession number NC_001806.1).
Results
Resistance of Clinical Isolates to Acyclovir
Herpes simplex virus type 1 isolates from an oral mucosal lesion, a swab sample of the right eye, and an oropharyngeal swab (D-1, D-2, and D-3, respectively) were phenotypically resistant to acyclovir 13 days into treatment (Table 1). Sequencing of the UL23 gene determined that these isolates were identical at both the amino acid level (Table 2) and at the nucleotide level (not shown). Relative to the reference sequence for HSV-1 strain 17, 13 nonsynonymous base-pair changes resulting in amino acid sequence changes were identified. Ten of these have been previously described and are not associated with acyclovir resistance. One (R41H) has been previously reported in both sensitive and resistant isolates [12–18], but whether this mutation has any role in resistance is not clear. Two mutations (A93V and Q261R) have not been previously reported as amino acid changes in either acyclovir-susceptible or -resistant strains (confirmed by searching GenBank). These occur outside the highly conserved regions of thymidine kinase, which include the adenosine triphosphate and nucleoside binding sites [19, 20]. Interestingly, the Q261R amino acid change is homologous to the same region of the HSV-2 thymidine kinase gene (based on the HG52 sequence, GenBank accession #NC_Z86099). It is therefore unlikely that this change is associated with acyclovir resistance. Notably, the A93V mutation was seen only in the 3 initial resistant isolates and not in the subsequent susceptible isolates D-4 and D-5. Resistant isolate D-6 also did not have the A93V mutation, but it was found to have the thymidine kinase mutation A189V, which has been previously associated with acyclovir resistance [14, 16, 20, 21].
Resistance of Clinical Isolates to Foscarnet and UL30 Sequencing
All clinical isolates were phenotypically susceptible to foscarnet (Table 3), and the frequency of resistant mutants remained low for these isolates despite the prolonged treatment with foscarnet. UL30 sequences from all clinical isolates were identical, and 5 nonsynonymous nucleotide changes were identified (Table 4). All amino acid changes identified were outside the regions of high DNA polymerase homology among Herpesviridae [12, 20, 22]. Of these, 4 have been previously reported as natural amino acid changes, and the P1199L amino acid change is at a site previously noted to have a different amino acid change not associated with resistance (P1199Q) [12]. This observation, combined with our observation that the P1199L amino acid change was found in both acyclovir-resistant and -susceptible isolates, suggests that this change is unlikely to be functionally significant from an antiviral standpoint.
Discussion
We describe the rapid selection for acyclovir-resistant HSV during treatment of likely primary HSV-1 infection in an immune-compromised patient in association with a previously undescribed amino acid change (A93V) in viral thymidine kinase. This strain was isolated from multiple sites within 2 weeks of initiation of antiviral treatment, but subsequent recurrences over the next 4 months were found to involve susceptible isolates. An ocular recurrence 5 months after initial infection was again phenotypically resistant, containing a different UL23 amino acid change (A189V) previously associated with resistance. Functional thymidine kinase assays using proteins with directed mutations are needed to validate the roles of both the A93V and A189V mutations in acyclovir resistance (eg, [23]).
Relatively rapid selection of resistant virus during therapy has been described after neonatal infection [24, 25] and in a pediatric patient with severe combined immune deficiency [26]. However, the majority of prior reports of resistance have occurred in the setting of prolonged and/or intermittent treatment with acyclovir for either recurrent or recalcitrant disease (reviewed in [2, 27]) and are largely concentrated on adult populations. An additional consideration in pediatric relative to adult patients is the increased likelihood of primary infection, as was suspected in this case. Although several decades of widespread acyclovir use in human populations have not been found to increase the background rate of acyclovir resistance [2, 28], there is likely to be increased peripheral and systemic replication of virus in individuals after primary infection (relative to reactivation), particularly in immune-compromised patients. Evidence for this is the detection of viremia even in immune-competent patients with primary or first clinical episode infection [29–31], which is not seen in otherwise healthy patients with HSV reactivation [31]. Our patient had prolonged systemic replication during treatment, with measureable DNAemia persisting >21 days after initiation of acyclovir. Given the low background rate of acyclovir resistance in HSV, it is quite unlikely that our patient had primary infection with a resistant strain, although such an occurrence has been described [32–34]. In those cases, primary infection with resistant virus was thought to have been acquired from an immune-compromised contact; our patient did not have a known contact with an individual who had HSV lesions prior to the presentation described in this report.
Selection of resistant virus by antiviral pressure prior to control of replication may have implications for latency and recurrence, likely increasing the chance that infected neurons may harbor resistant virus and compromising or confusing treatment choices after any subsequent reactivations. This is illustrated in our case with the oral reactivation on day 102 (D-5), which was treated with foscarnet pending phenotypic resistance testing, results of which can take several weeks to obtain. We also noted reactivation of an acyclovir-resistant strain in the eye on day 144 (D-6), for which acyclovir was continued. This reactivation resolved despite continuation of acyclovir, likely due to corneal epithelial debridement to reduce the overall amount of virus in the eye and in part to topical trifluorothymidine. Improvement in patient immunity may have also played a role (the increased lymphocyte count noted following recovery from the last cycle of chemotherapy may also have been accompanied by improved lymphocyte function, Figure 1); however, specific antiviral immunity (including seroconversion) was not assessed in this patient after apparent control of this infection.
The difficulty of treating acyclovir-resistant ocular disease is also highlighted by our case. Acyclovir-resistant keratitis is common even in immune-competent patients and has been attributed to frequent and chronic treatment with topical acyclovir [7, 16]. These studies also noted that ocular recurrences may have variable resistance patterns at different times in the same patient, as we observed in this report (ocular isolates D-2, D-4, and D-6). In addition to supporting prior observations that acyclovir-resistant virus is capable of reactivating in humans, our case suggests the possibility that limited penetration of corneal tissue and/or trigeminal ganglia by antivirals and perhaps also surveilling anti-HSV lymphocytes may increase the chances of reactivation of a resistant clone of virus.
From a clinical standpoint, our case also illustrates understudied aspects of the treatment of HSV infection in compromised patients. Current treatment regimens commonly use a single agent (usually acyclovir); however, given the observations that primary infection may result in higher initial replication of virus, most viral isolates contain a low but measureable amount of acyclovir-resistant clones, and immune-compromised patients are more likely to have clinically significant disease with acyclovir-resistant HSV, strong consideration could be given to initial combination treatment when primary infection is suspected in a compromised patient. Although medication toxicities (particularly nephrotoxicity with foscarnet) are of concern with such an approach, pediatric patients, relative to adults, are less likely to have pre-existing underlying renal disease, and this population may better tolerate a combination of acyclovir with foscarnet. A clinical study to test the potential benefit of this approach would be difficult to design and likely to require multicenter cooperation. Given the low toxicity and minimal drug interactions of acyclovir, consideration could also be given to acyclovir prophylaxis in heavily immune-suppressed HSV-seronegative pediatric patients. Alternative antiviral therapy could also be considered. Although cidofovir has demonstrated efficacy in vitro and there are case reports of successful use in vivo [35, 36], its use may be limited by nephrotoxicity, difficult dosing, questionable penetration into the central nervous system [17], and unclear efficacy for HSV [37–39]. Antivirals for HSV with different mechanisms of action than those in current use represent an important medical need [20, 32, 40].
Lastly, our report highlights the importance of testing isolates for resistance in immune-compromised patients and supports a need for more rapid testing strategies (see [40, 41]). Sequencing isolates has the advantage of providing rapid results (eg, [18]), but is limited by lack of a complete understanding of amino acid changes leading to resistance, including the previously undescribed thymidine kinase A93V amino acid change we report here (which may or may not directly lead to acyclovir resistance). Most hospital laboratories are not equipped for phenotypic resistance testing and require samples to be sent to an outside laboratory.
Supplementary Data
Supplementary materials are available at the Journal of the Pediatric Infectious Diseases Society online (http://jpids.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Acknowledgments
The authors acknowledge the technical assistance of Denise Wagner and Nanette Susmarski in isolating, growing, and titering the clinical isolates. This project benefited from the scientific advice of Anne Rowley, Patricia Spear, and Richard Longnecker. The control virus I-309 was originally obtained by Patricia Spear from Bernard Roizman (who generously provided permission for its use), and control strain 615.8 was a generous gift from Don Coen. Traditional sequencing services were performed at the Northwestern University Genomics Core Facility.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (5K08 AI089942 to W. J. M.).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form of Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Schiffer JT, Corey L. Herpes simplex virus. In: Mandell GL, Bennett JE, Dolin R, editors. Mandell, Douglas, and Bennett's principles and practice of infectious diseases. 7th. Philadelphia: Churchill Livingstone; 2009. p. 1948. [Google Scholar]
- 2.Bacon TH, Levin MJ, Leary JJ, Sarisky RT, Sutton D. Herpes simplex virus resistance to acyclovir and penciclovir after two decades of antiviral therapy. Clin Microbiol Rev. 2003;16:114–28. doi: 10.1128/CMR.16.1.114-128.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sarisky RT, Nguyen TT, Duffy KE, Wittrock RJ, Leary JJ. Difference in incidence of spontaneous mutations between herpes simplex virus types 1 and 2. Antimicrob Agents Chemother. 2000;44:1524–9. doi: 10.1128/aac.44.6.1524-1529.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shin YK, Cai GY, Weinberg A, Leary JJ, Levin MJ. Frequency of acyclovir-resistant herpes simplex virus in clinical specimens and laboratory isolates. J Clin Microbiol. 2001;39:913–7. doi: 10.1128/JCM.39.3.913-917.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coen DM. Acyclovir-resistant, pathogenic herpesviruses. Trends Microbiol. 1994;2:481–5. doi: 10.1016/0966-842x(94)90652-1. [DOI] [PubMed] [Google Scholar]
- 6.Field HJ. Herpes simplex virus antiviral drug resistance—current trends and future prospects. J Clin Virol. 2001;21:261–9. doi: 10.1016/s1386-6532(00)00169-4. [DOI] [PubMed] [Google Scholar]
- 7.Duan R, de Vries RD, Osterhaus AD, Remeijer L, Verjans GM. Acyclovir-resistant corneal HSV-1 isolates from patients with herpetic keratitis. J Infect Dis. 2008;198:659–63. doi: 10.1086/590668. [DOI] [PubMed] [Google Scholar]
- 8.Mocarski ES, Post LE, Roizman B. Molecular engineering of the herpes simplex virus genome: insertion of a second L-S junction into the genome causes additional genome inversions. Cell. 1980;22:243–55. doi: 10.1016/0092-8674(80)90172-5. [DOI] [PubMed] [Google Scholar]
- 9.Sacks SL, Wanklin RJ, Reece DE, Hicks KA, Tyler KL, Coen DM. Progressive esophagitis from acyclovir-resistant herpes simplex. Clinical roles for DNA polymerase mutants and viral heterogeneity? Ann Intern Med. 1989;111:893–9. doi: 10.7326/0003-4819-111-11-893. [DOI] [PubMed] [Google Scholar]
- 10.Safrin S, Elbeik T, Phan L, et al. Correlation between response to acyclovir and foscarnet therapy and in vitro susceptibility result for isolates of herpes simplex virus from human immunodeficiency virus-infected patients. Antimicrob Agents Chemother. 1994;38:1246–50. doi: 10.1128/aac.38.6.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Safrin S, Kemmerly S, Plotkin B, et al. Foscarnet-resistant herpes simplex virus infection in patients with AIDS. J Infect Dis. 1994;169:193–6. doi: 10.1093/infdis/169.1.193. [DOI] [PubMed] [Google Scholar]
- 12.Burrel S, Deback C, Agut H, Boutolleau D. Genotypic characterization of UL23 thymidine kinase and UL30 DNA polymerase of clinical isolates of herpes simplex virus: natural polymorphism and mutations associated with resistance to antivirals. Antimicrob Agents Chemother. 2010;54:4833–42. doi: 10.1128/AAC.00669-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sauerbrei A, Deinhardt S, Zell R, Wutzler P. Phenotypic and genotypic characterization of acyclovir-resistant clinical isolates of herpes simplex virus. Antiviral Res. 2010;86:246–52. doi: 10.1016/j.antiviral.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Chibo D, Druce J, Sasadeusz J, Birch C. Molecular analysis of clinical isolates of acyclovir resistant herpes simplex virus. Antiviral Res. 2004;61:83–91. doi: 10.1016/j.antiviral.2003.08.018. [DOI] [PubMed] [Google Scholar]
- 15.Bestman-Smith J, Schmit I, Papadopoulou B, Boivin G. Highly reliable heterologous system for evaluating resistance of clinical herpes simplex virus isolates to nucleoside analogues. J Virol. 2001;75:3105–10. doi: 10.1128/JVI.75.7.3105-3110.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duan R, de Vries RD, van Dun JM, et al. Acyclovir susceptibility and genetic characteristics of sequential herpes simplex virus type 1 corneal isolates from patients with recurrent herpetic keratitis. J Infect Dis. 2009;200:1402–14. doi: 10.1086/606028. [DOI] [PubMed] [Google Scholar]
- 17.Schulte EC, Sauerbrei A, Hoffmann D, Zimmer C, Hemmer B, Muhlau M. Acyclovir resistance in herpes simplex encephalitis. Ann Neurol. 2010;67:830–3. doi: 10.1002/ana.21979. [DOI] [PubMed] [Google Scholar]
- 18.Bohn K, Zell R, Schacke M, Wutzler P, Sauerbrei A. Gene polymorphism of thymidine kinase and DNA polymerase in clinical strains of herpes simplex virus. Antivir Ther. 2011;16:989–97. doi: 10.3851/IMP1852. [DOI] [PubMed] [Google Scholar]
- 19.Morfin F, Souillet G, Bilger K, Ooka T, Aymard M, Thouvenot D. Genetic characterization of thymidine kinase from acyclovir-resistant and -susceptible herpes simplex virus type 1 isolated from bone marrow transplant recipients. J Infect Dis. 2000;182:290–3. doi: 10.1086/315696. [DOI] [PubMed] [Google Scholar]
- 20.Piret J, Boivin G. Resistance of herpes simplex viruses to nucleoside analogues: mechanisms, prevalence, and management. Antimicrob Agents Chemother. 2011;55:459–72. doi: 10.1128/AAC.00615-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stranska R, van Loon AM, Polman M, et al. Genotypic and phenotypic characterization of acyclovir-resistant herpes simplex viruses isolated from haematopoietic stem cell transplant recipients. Antivir Ther. 2004;9:565–75. [PubMed] [Google Scholar]
- 22.Schmit I, Boivin G. Characterization of the DNA polymerase and thymidine kinase genesof herpes simplex virus isolates from AIDS patients in whom acyclovirand foscarnet therapy sequentially failed. J Infect Dis. 1999;180:487–90. doi: 10.1086/314900. [DOI] [PubMed] [Google Scholar]
- 23.Frobert E, Ooka T, Cortay JC, Lina B, Thouvenot D, Morfin F. Herpes simplex virus thymidine kinase mutations associated with resistance to acyclovir: a site-directed mutagenesis study. Antimicrob Agents Chemother. 2005;49:1055–9. doi: 10.1128/AAC.49.3.1055-1059.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oram RJ, Marcellino D, Strauss D, et al. Characterization of an acyclovir-resistant herpes simplex virus type 2 strain isolated from a premature neonate. J Infect Dis. 2000;181:1458–61. doi: 10.1086/315387. [DOI] [PubMed] [Google Scholar]
- 25.Levin MJ, Weinberg A, Leary JJ, Sarisky RT. Development of acyclovir-resistant herpes simplex virus early during the treatment of herpes neonatorum. Pediatr Infect Dis J. 2001;20:1094–7. doi: 10.1097/00006454-200111000-00021. [DOI] [PubMed] [Google Scholar]
- 26.Sibrack CD, Gutman LT, Wilfert CM, et al. Pathogenicity of acyclovir-resistant herpes simplex virus type 1 from an immunodeficient child. J Infect Dis. 1982;146:673–82. doi: 10.1093/infdis/146.5.673. [DOI] [PubMed] [Google Scholar]
- 27.Morfin F, Thouvenot D. Herpes simplex virus resistance to antiviral drugs. J Clin Virol. 2003;26:29–37. doi: 10.1016/s1386-6532(02)00263-9. [DOI] [PubMed] [Google Scholar]
- 28.Danve-Szatanek C, Aymard M, Thouvenot D, et al. Surveillance network for herpes simplex virus resistance to antiviral drugs: 3-year follow-up. J Clin Microbiol. 2004;42:242–9. doi: 10.1128/JCM.42.1.242-249.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diamond C, Mohan K, Hobson A, Frenkel L, Corey L. Viremia in neonatal herpes simplex virus infections. Pediatr Infect Dis J. 1999;18:487–9. doi: 10.1097/00006454-199906000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Johnston C, Magaret A, Selke S, Remington M, Corey L, Wald A. Herpes simplex virus viremia during primary genital infection. J Infect Dis. 2008;198:31–4. doi: 10.1086/588676. [DOI] [PubMed] [Google Scholar]
- 31.Juhl D, Mosel C, Nawroth F, et al. Detection of herpes simplex virus DNA in plasma of patients with primary but not with recurrent infection: implications for transfusion medicine? Transfus Med. 2010;20:38–47. doi: 10.1111/j.1365-3148.2009.00951.x. [DOI] [PubMed] [Google Scholar]
- 32.Kost RG, Hill EL, Tigges M, Straus SE. Brief report: recurrent acyclovir-resistant genital herpes in an immunocompetent patient. N Engl J Med. 1993;329:1777–82. doi: 10.1056/NEJM199312093292405. [DOI] [PubMed] [Google Scholar]
- 33.Jones TJ, Paul R. Disseminated acyclovir-resistant herpes simplex virus type 2 treated successfully with foscarnet. J Infect Dis. 1995;171:508–9. doi: 10.1093/infdis/171.2.508. [DOI] [PubMed] [Google Scholar]
- 34.Safrin S. Acyclovir-resistant herpes simplex virus infection. J Infect Dis. 1995;172:603. doi: 10.1093/infdis/172.2.603. [DOI] [PubMed] [Google Scholar]
- 35.Snoeck R, Andrei G, Gerard M, et al. Successful treatment of progressive mucocutaneous infection due to acyclovir- and foscarnet-resistant herpes simplex virus with (S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine (HPMPC) Clin Infect Dis. 1994;18:570–8. doi: 10.1093/clinids/18.4.570. [DOI] [PubMed] [Google Scholar]
- 36.LoPresti AE, Levine JF, Munk GB, Tai CY, Mendel DB. Successful treatment of an acyclovir- and foscarnet-resistant herpes simplex virus type 1 lesion with intravenous cidofovir. Clin Infect Dis. 1998;26:512–3. doi: 10.1086/517101. [DOI] [PubMed] [Google Scholar]
- 37.Dvorak CC, Cowan MJ, Horn B, Weintrub PS. Development of herpes simplex virus stomatitis during receipt of cidofovir therapy. Clin Infect Dis. 2009;49:e92–5. doi: 10.1086/605678. [DOI] [PubMed] [Google Scholar]
- 38.Wyles DL, Patel A, Madinger N, Bessesen M, Krause PR, Weinberg A. Development of herpes simplex virus disease in patients who are receiving cidofovir. Clin Infect Dis. 2005;41:676–80. doi: 10.1086/432477. [DOI] [PubMed] [Google Scholar]
- 39.Chen Y, Scieux C, Garrait V, et al. Resistant herpes simplex virus type 1 infection: an emerging concern after allogeneic stem cell transplantation. Clin Infect Dis. 2000;31:927–35. doi: 10.1086/314052. [DOI] [PubMed] [Google Scholar]
- 40.Wang Y, Wang Q, Zhu Q, Zhou R, Liu J, Peng T. Identification and characterization of acyclovir-resistant clinical HSV-1 isolates from children. J Clin Virol. 2011;52:107–12. doi: 10.1016/j.jcv.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 41.Frobert E, Cortay JC, Ooka T, et al. Genotypic detection of acyclovir-resistant HSV-1: characterization of 67 ACV-sensitive and 14 ACV-resistant viruses. Antiviral Res. 2008;79:28–36. doi: 10.1016/j.antiviral.2008.01.153. [DOI] [PubMed] [Google Scholar]
- 42.Morfin F, Thouvenot D, Aymard M, Souillet G. Reactivation of acyclovir-resistant thymidine kinase-deficient herpes simplex virus harbouring single base insertion within a 7 Gs homopolymer repeat of the thymidine kinase gene. J Med Virol. 2000;62:247–50. [PubMed] [Google Scholar]
- 43.Kudo E, Shiota H, Naito T, Satake K, Itakura M. Polymorphisms of thymidine kinase gene in herpes simplex virus type 1: analysis of clinical isolates from herpetic keratitis patients and laboratory strains. J Med Virol. 1998;56:151–8. doi: 10.1002/(sici)1096-9071(199810)56:2<151::aid-jmv9>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]