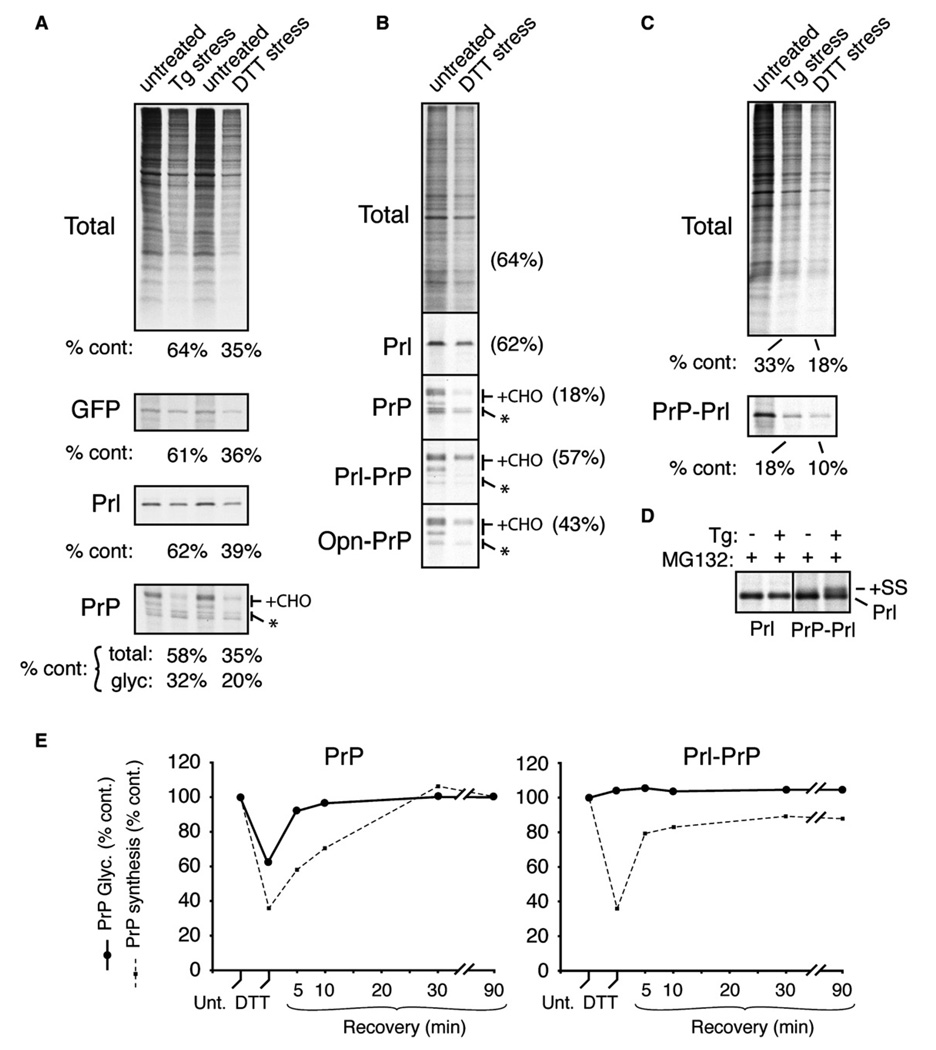
Figure 1. Signal Sequence-Specific Translocational Attenuation of PrP during Acute Stress.
(A) Immunoprecipitation of transfected products (GFP, Prl, or PrP) from pulse-labeled (15 min) cultured HeLa cells treated for 30 min with 10 µM Tg or 10 mM DTT. Radiolabeled products recovered from stressed cells were quantified relative to untreated cells. For PrP, the amount of glycosylated species (+CHO) was also quantified separately. Asterisk is unglycosylated PrP.
(B) The indicated constructs were analyzed as in (A), except COS-7 cells were used, labeling was for 10 min, and 100 µM ALLN (a proteasome inhibitor) was included.
(C) PrP-Prl was analyzed and quantified as in (A).
(D) Prl and PrP-Prl were immunoprecipitated from pulse-labeled transfected cells treated with 10 µM Tg in the presence of 10 µM MG132 (a proteasome inhibitor) as indicated. Note that PrP-Prl (but not Prl) generates signal sequence-containing precursor (+SS, indicative of nontranslocated protein) in a stress-dependent manner, illustrating its translocational attenuation.
(E) Time course of recovery from translocational attenuation. COS-7 cells that were either untreated, acutely treated (30 min) with 10 mM DTT, or recovered for between 5 and 90 min were pulse labeled for 10 min and analyzed by immunoprecipitation of PrP. The efficiencies of glycosylation (solid line) and synthesis (dashed line) of either PrP (left graph) or Prl-PrP (right graph) relative to untreated cells are plotted.