Fig. 7.
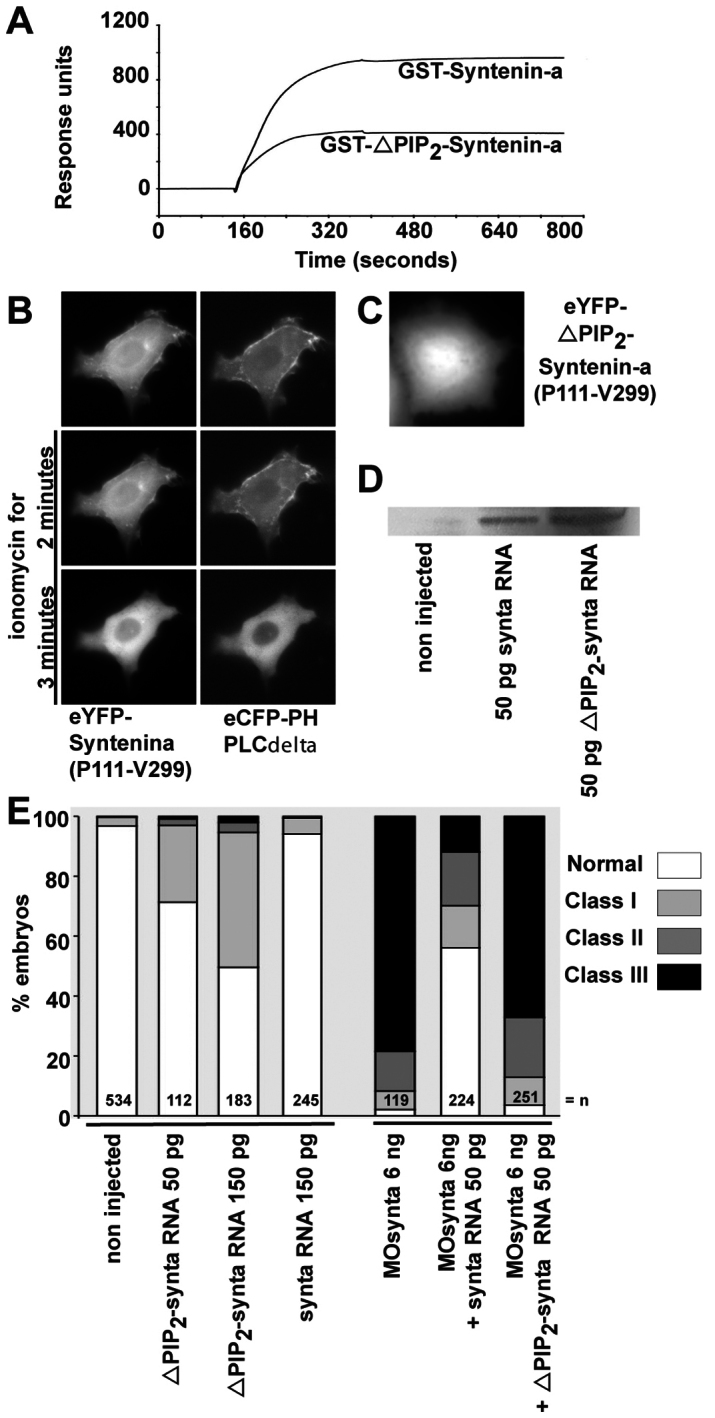
Syntenin-a interaction with PIP2 is required for epiboly progression. (A) SPR experiment showing the binding of syntenin-a, and the reduced binding of the syntenin-a PDZ1 mutant (ΔPIP2–syntenin-a), to PIP2-containing liposomes. Proteins were perfused at 0.5 μM. (B) Time-lapse microscopy of a MCF-7 cell, before and after ionomycin-induced plasma membrane PIP2 breakdown. The cell overexpressed the PDZ tandem and C-terminal domain of syntenin-a (P111-V299) fused to eYFP, and the PH domain of PLC∂ fused to eCFP. Syntenin-a (P111-V299) colocalizes with PH-PLC∂, and both fluorescent signals delocalize simultaneously from the plasma membrane after PIP2 breakdown, indicative of a syntenin-a–PIP2 interaction in a cellular context. (C) Micrograph of a MCF-7 cell expressing syntenin-a (Pro111-Val299) with a mutant PDZ1 domain deficient for PIP2 binding, fused to eYFP, showing no plasma membrane enrichment. (D) Western blot showing that injection of 50 pg of RNA encoding either wild-type or ΔPIP2–syntenin-a yields similar levels of protein expression. (E) Left part, percentage of embryos found in each class (as in Fig. 1G) upon injection of RNA encoding syntenin-a deficient for PIP2 interaction (ΔPIP2-synta) or wild-type syntenin-a (synta). Right bars show percentage of embryos found in each class upon injection of MOsynta, together with wild-type or ΔPIP2–syntax. Note that ΔPIP2-synta expression affects epiboly by itself, and has no rescue potential.
