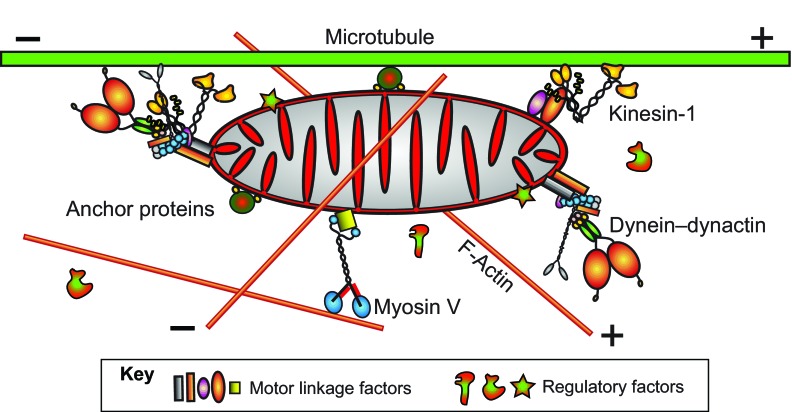
Fig. 2.
Axonal transport machinery for mitochondria. Plus- and minus-end-directed motion of mitochondria along axonal microtubules is driven by kinesin-1 and dynein, respectively. Forces from cytoplasmic myosins (e.g. myosin V) might modulate the processivity of microtubule-based long-range transport and might drive short-range local transport. There are many important questions about mitochondria transport mechanisms that need to be addressed. How are motors linked to mitochondria and how are those linkages controlled? How are motor–filament interactions controlled? What are the functional and physical relationships between motors; e.g. are different types of motors joined in multi-motor complexes (left end) or are they physically separate (right end)? Static anchorage complexes that help hold mitochondria in a stationary state can suppress motor-driven movements (center). What is the nature of those anchorage complexes and how are they controlled? Substantial progress has been made and answers are beginning to emerge. Identified linkers for kinesin-1 so far include Miro, Milton and syntabulin, and they probably have additional interacting proteins that make important contributions. One identified anchor complex includes syntaphilin and LC8, but others are expected to exist. Regulatory factors that influence linkage and motor function include Ca2+ and a variety of cytoplasmic and mitochondrial proteins, including kinases, tau and Miro.