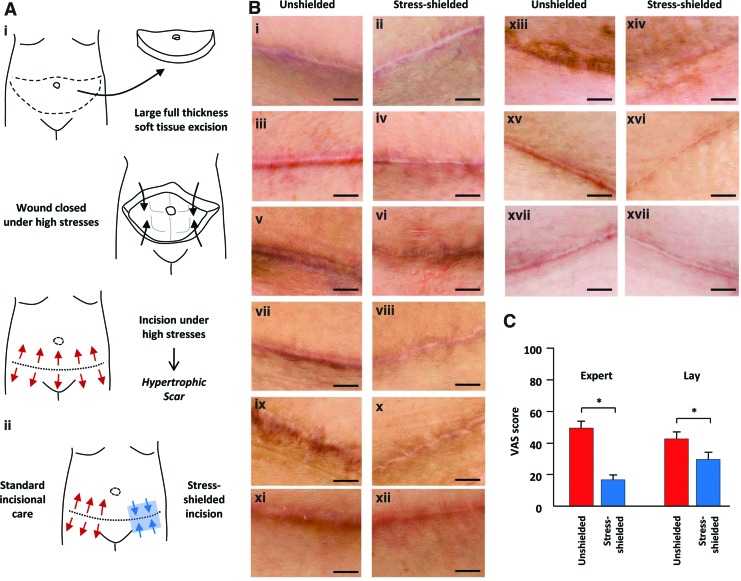
Figure 5.
Phase I clinical trial in abdominal surgery patients. (A) Schematic of abdominoplasty surgery (i) involving the excision of skin and subcutaneous fat and surgical closure under high mechanical forces that predispose wounds to robust scar formation. Clinical study schematic (ii) demonstrates application of the stress-shielding polymer to one side of the incision, whereas the within-patient control side is left unshielded. (B) Photographs of paired abdominal incisions at 6–12 months postsurgery (paired rows). Note the significant scar elevation, widening, discoloration, and irregularity in unshielded control incisions (i, iii, v, vii, ix, xi, xiii, xv, xvii) compared with device stress-shielded treatment incisions (ii, iv, vi, viii, x, xii, xiv, xvi, xviii). (C) Quantification of expert and lay panel analyses using a visual analog scale (VAS), with lower scores indicating improved scar appearance. Data are means±standard errors of the mean. Scale bar is 1 cm. *p<0.01. Figure reprinted with permission from Gurtner et al.30 To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound