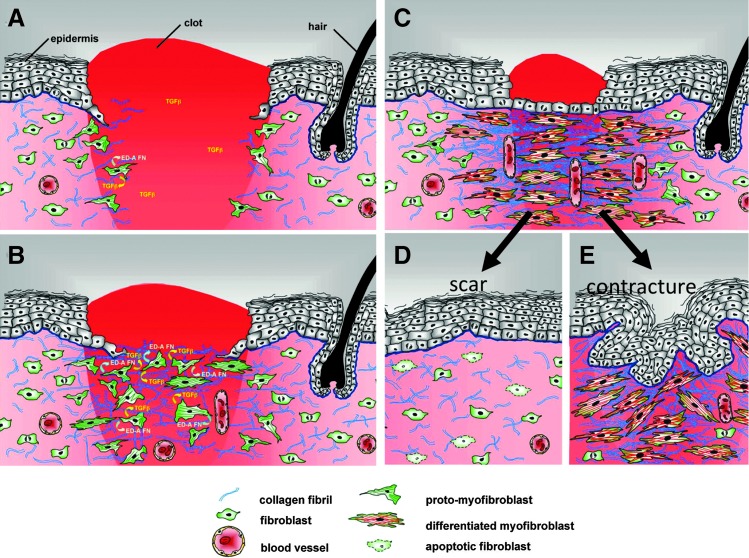
Figure 5.
Model of myofibroblast differentiation and wound contraction. (A) In normal tissues, fibroblasts are shielded from “routine” external mechanical perturbations in the skin by the collagen-rich ECM that they have assembled, such that the organization of a contractile cytoskeleton is not stimulated (light pink area of dermis). Following a full-thickness dermal injury, the wound is filled with a provisional matrix comprised of fibrin and fibronectin and a complement of newly released growth factors and cytokines. Fibroblasts, along with blood vessels, are stimulated to migrate into this pro-migration microenvironment and over time replace the provisional matrix with an ECM comprised of collagen and cellular FNs to form granulation tissue. (B) Tractional forces accompanying fibroblast migration are responsible for local areas of increased stiffness in newly made collagen. Focal adhesion assembly is increased with increasing stiffness and this is accompanied by clustering of integrins within focal adhesions and increased stress fiber assembly resulting in fibroblast acquisition of the proto-myofibroblast phenotype. Tensional forces and growth factors stimulate proto-myofibroblasts to secrete transforming growth factor-beta1 (TGF-β1) and increased levels of ED-A FN. (C) In response to TGF-β1, a threshold of mechanical stiffness and the presence of specific isoforms of FN proto-myofibroblasts differentiate into myofibroblasts. Feed-forward pathways, including TGF-β1, the mechanical environment, actin dynamics, SRF/MRTF transcriptional activation, and Hic-5, are responsible for myofibroblast function and persistence. At the same time, differentiated myofibroblasts deposit collagen and other ECM components, and produce proteases. This complex process of remodeling results in shortening of the collagen matrix with corresponding wound closure. (D) During normal acute wound healing, these feed-forward mechanisms are temporally limited and myofibroblast numbers diminish as a result of apoptosis and/or senescence. (E) In pathological situations, such as HTS formation, these feed-forward pathways presumably persist allowing for continued myofibroblast presence resulting in continued ECM deposition and remodeling. In conclusion, myofibroblasts, far from being a “bad” cell type, are functionally essential cells. It is their dysregulation that is the cause of tissue dysfunction. (Modified from Fig. 9, Tomasek et al.2). FN, fibronectins; SRF, serum response factor; MRTFA/B, myocardin-related transcription factor A/B.