Abstract
Background
The purpose of this study was to examine whether long-term use of anticoagulants in elderly patients with atrial fibrillation (AF) and chronic kidney disease (CKD) influences renal function.
Methods
In this retrospective observational study, we reviewed the records of 2023 patients who attended our institution for treatment of CKD between January 2001 and September 2012. Inclusion criteria were having been under review for three months or more, age older than 60 years, permanent AF, a CHADS2 score > 2, and National Kidney Foundation Kidney Disease Outcomes Quality Initiative CKD stage 3–5. Sixty-one patients fulfilled these criteria, and were divided into those receiving antiplatelet anticoagulation (group A) and those receiving warfarin (group B). The results of laboratory investigations and estimated glomerular filtration rate (GFR) were recorded at months 3, 6, 12, and 18 from treatment initiation. We also recorded the occurrence of serious cardiovascular and neurological events, significant bleeding, and survival beyond 12 years.
Results
Of the 61 patients enrolled, 35 were in group A and 26 were in group B. The mean international normalized ratio (INR) was 1.95 ± 1.01 (goal < 3.0). After adjustment for potential confounding variables, we found that patients in group B had a higher estimated GFR (6.06 ± 2.36 mL per minute, P = 0.01). Over a 12-year observation period, group B patients had significantly (P = 0.013) better survival than group A, with an adjusted hazard ratio for mortality of 0.318 (P = 0.022).
Conclusion
Warfarin therapy may delay deterioration in renal function and improve survival of elderly patients with CKD and AF.
Keywords: aged, atrial fibrillation, chronic kidney disease, vitamin K antagonists, warfarin
Introduction
Chronic kidney disease (CKD) is a growing problem due to our aging population and their coexisting comorbidities.1 Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia in patients with CKD,2 is the most common arrhythmia requiring medical therapy, and its prevalence increases with advancing age.3 New-onset AF also occurs with aging as the glomerular filtration rate (GFR) declines and proteinuria worsens.4,5 Approximately 15% of patients are found to have CKD when AF is diagnosed.6 Go et al have estimated that, in the United States alone, more 5.6 million people will be affected by AF by 2050, with more than half those affected aged 80 years or more.7
The Framingham study showed that AF is an independent risk factor for stroke.8 Almost 90% of deaths caused by stroke occur in people over 65 years of age. Contemporary therapy for AF aims to relieve symptoms and prevent complications such as stroke and heart failure.9 In clinical practice, the CHADS2 score (congestive heart failure, 1 point; hypertension, 1 point; ≥75 years old, 1 point; diabetes, 1 point; prior stroke or transient ischemic attack or thromboembolism, 2 points) is a predictor for estimating the risk of stroke in patients with AF10 and is used to determine whether oral anticoagulation, typically with vitamin K antagonists, is required.
AF in patients with CKD and a high CHADS2 score (≥2 points) is associated with a poor outcome and vitamin K antagonist therapy is recommended to reduce mortality and the risk of stroke.11–13 Randomized controlled trials have shown that prophylactic treatment with warfarin decreases the risk of stroke by 60%–70% in patients with AF.14 However, although the risk of stroke increases with age, so does the risk of hemorrhage secondary to anticoagulation; as a result, elderly patients tend to be undertreated with warfarin.15 In addition, warfarin appears to be capable of causing direct renal injury, with warfarin-related nephropathy being reported in patients with and without CKD.16
The primary objective of this study was to assess the impact of warfarin therapy on estimated GFR in elderly patients with CKD and AF. Our secondary objective was to assess the impact of warfarin therapy on all-cause mortality in these patients.
Materials and methods
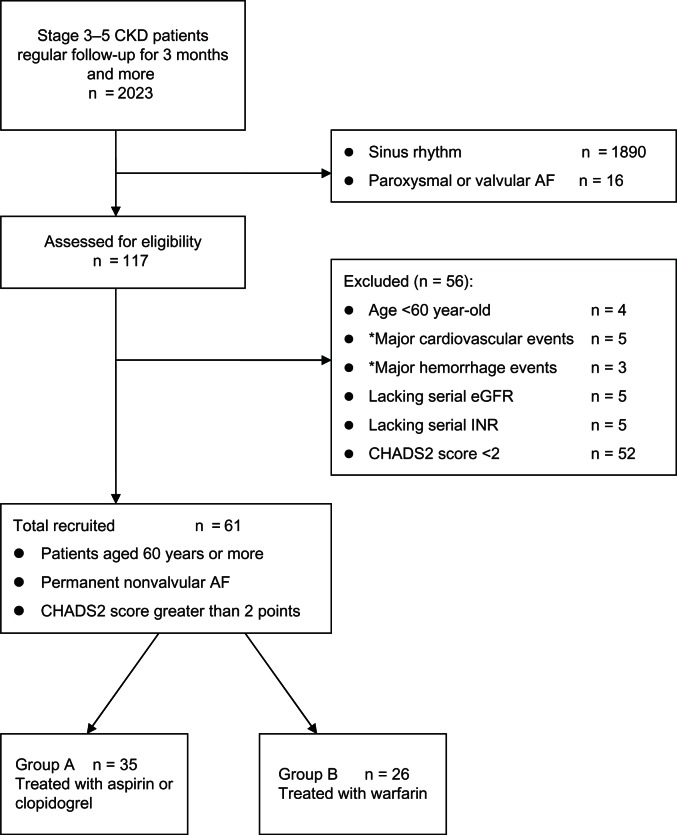
We performed a retrospective cohort study, examining the records of 2023 patients with CKD treated in our tertiary medical center between January 2001 and September 2012. From these records, we selected a cohort of patients aged 60 years or more, in permanent nonvalvular AF with a CHADS2 score greater than 2 points, National Kidney Foundation Kidney Disease Outcomes Quality Initiative17 CKD stage 3–5, and under regular follow-up for more than three months. We excluded patients lacking serial estimated GFR data or lacking regular international normalized ratio (INR) measurements and those with cardiovascular or major hemorrhage events at first hospitalization. To improve the quality of reporting observational studies, the manuscript was organized in a manner compliant with the STROBE (The Strengthening the Reporting of Observational Studies in Epidemiology) statement.18 Patient flow is show in Figure 1, including the number of patients screened and excluded. The formula used for estimated GFR was a simplified Modification of Diet in Renal Disease equation:
Figure 1.
Participant flow diagram depicting screening/enrollment process.
Note: *Events at first hospitalization.
Abbreviations: CKD, chronic kidney disease; AF, atrial fibrillation; CHADS2 score, congestive heart failure (1 point), hypertension (1 point), ≥75 years old (1 point), diabetes (1 point), prior stroke or transient ischemic attack or thromboembolism (2 points); eGFR, estimated glomerular filtration rate; INR, international normalized ratio.
During the study, despite the small sample, we made an effort to maintain representativeness and to ensure generality. We checked the INR for patients commenced on warfarin every two weeks in the first month, monthly for three months, and then once every three months. The target range for INR was 2–3 and the usual dose of warfarin was 1–2.5 mg daily. AF was diagnosed by standard 12-lead or Holter electrocardiography regardless of symptoms. Diagnoses of paroxysmal, persistent, or permanent AF were made according to American College of Cardiology, American Heart Association, and European Society of Cardiology guidelines.19
The 61 patients selected were divided into two groups: those receiving an antiplatelet regimen such as aspirin 100 mg/day or clopidogrel 75 mg/day but not warfarin (group A) and those receiving warfarin treatment (group B). We used the Kidney Disease Outcomes Quality Initiative guidelines to determine the goals for ideal blood pressure, glucose, and lipid control. The protocol was approved by our institutional review board. All enrolled subjects provided their written informed consent.
Statistical analysis
The Mann-Whitney U test was used to compare the median values of continuous variables between the groups. The Wilcoxon signed rank test was used to examine within-group changes at different time points. The Chi-square test or Fisher’s Exact test was used to compare categorical data. To estimate the effect of warfarin treatment and adjust for the possible inter-correlations of data collected from the same patient and other covariates, we used a generalized estimating equation to fit a linear regression model that yields robust standard errors estimated in the model. Survival analysis was evaluated by the Kaplan-Meier method and the log rank test; variables that achieved statistical significance (P < 0.05) in univariate analysis were subsequently subject to multivariate analysis using Cox proportional hazards analysis. Corresponding hazard ratios are reported along with their 95% confidence intervals (CI). Two-sided tests were used at the significance level of 5%, and all analyses were performed using the Statistical Package for Social Sciences version 15.0 (SPSS Inc, Chicago, Il, USA).
Results
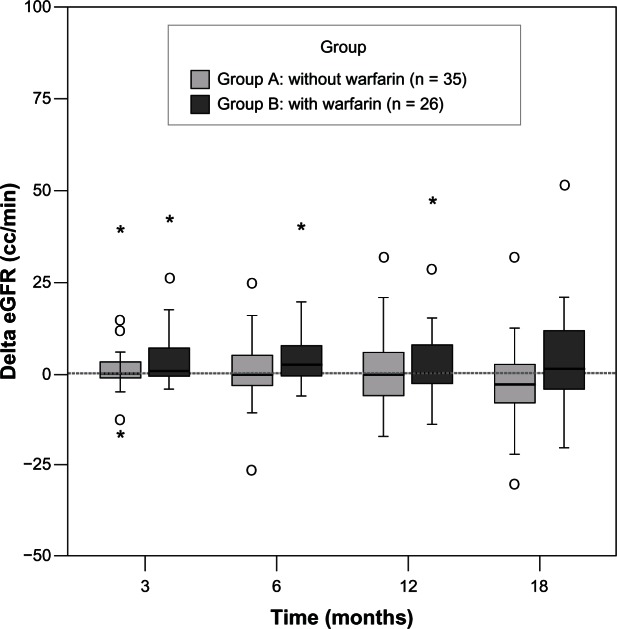
Of the 61 patients selected for the study, 35 were maintained on aspirin or clopidogrel (12 women and 23 men, of median age 79.9 years, range 60.1–96.8, group A) and the remainder were treated with warfarin (10 women and 16 men, median age 73.4 years, range 60.1–88.5, group B). F baseline renal function between those treated with warfarin There were 27 with diabetes, 35 with hypertension, 21 with glomerulonephritis (not biopsy-proven), and five with gouty nephropathy. There were no statistically significant differences in gender or age between the groups. There were also no significant differences in biochemistry findings or baseline renal function between those treated with warfarin and those who were not (Table 1). The mean INR was 1.95 ± 1.01 (goal 2.0–3.0). Median estimated GFR increased over time in group B. There was no significant difference in the change in estimated GFR between the groups at any time point, but there was a trend suggesting that renal function was better in group B (Figure 2). When estimating the effect of warfarin treatment over 18 months, estimated GFR was better preserved in patients taking warfarin (by 6.06 ± 2.36 mL per minute, P = 0.01, Table 2). We also noted that patients’ renal function had a significant negative correlation with stage 5 CKD, low-density lipoprotein, and serum calcium. During 18 months of observation, no patient in either group needed dialysis.
Table 1.
Categorical comparison of data in enrolled age, baseline renal function, and biochemistry between group A and group B
| Group
|
P value | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A (without warfarin)
|
B (with warfarin)
|
||||||||||||
| n | Mean | SD | Median | Min | Max | n | Mean | SD | Median | Min | Max | ||
| Age at onset of AF | 33 | 79.66 | 7.41 | 79.91 | 60.08 | 96.83 | 26 | 73.97 | 7.67 | 73.41 | 58.71 | 88.45 | 0.006 |
| Age at study enrollment | 35 | 78.71 | 6.48 | 78.52 | 61.29 | 94.37 | 26 | 75.35 | 6.79 | 77.25 | 62.39 | 85.17 | 0.093 |
| eGFR, baseline | 35 | 23.12 | 11.05 | 22.74 | 6.91 | 48.42 | 26 | 27.96 | 13.15 | 26.68 | 4.94 | 64.34 | 0.175 |
| BUN | 23 | 41.70 | 21.51 | 33.70 | 18.00 | 89.00 | 16 | 40.44 | 20.92 | 32.50 | 13.90 | 81.30 | 0.819 |
| Albumin | 16 | 3.61 | 0.88 | 4.05 | 1.50 | 4.50 | 14 | 3.80 | 0.58 | 3.95 | 2.60 | 4.60 | 0.173 |
| Cholesterol | 28 | 158.04 | 41.82 | 163.00 | 85.00 | 237.00 | 18 | 181.06 | 51.82 | 171.50 | 97.00 | 278.00 | 0.196 |
| Triglycerides | 26 | 119.92 | 81.26 | 98.00 | 41.00 | 384.00 | 17 | 134.35 | 94.93 | 121.00 | 36.00 | 467.00 | 0.970 |
| HDL | 18 | 48.17 | 10.61 | 46.00 | 30.00 | 69.00 | 14 | 47.43 | 10.38 | 44.50 | 32.00 | 71.00 | 0.309 |
| LDL | 19 | 89.20 | 33.01 | 86.00 | 37.00 | 183.00 | 13 | 107.14 | 43.29 | 92.00 | 54.00 | 194.00 | 0.181 |
| Uric acid | 26 | 8.24 | 2.24 | 7.85 | 4.30 | 14.10 | 18 | 7.38 | 2.29 | 7.15 | 4.30 | 12.00 | 0.056 |
| Hemocrit | 29 | 31.04 | 6.04 | 30.50 | 21.40 | 45.50 | 19 | 34.59 | 6.99 | 34.90 | 20.20 | 46.20 | 0.067 |
| Sodium | 21 | 137.24 | 3.45 | 138.00 | 130.00 | 142.00 | 14 | 138.79 | 4.81 | 139.00 | 124.00 | 145.00 | 0.897 |
| Potassium | 28 | 4.27 | 0.71 | 4.20 | 3.00 | 6.10 | 17 | 4.12 | 0.64 | 4.40 | 3.00 | 5.20 | 0.188 |
| Calcium | 14 | 8.35 | 0.60 | 8.35 | 7.40 | 9.30 | 11 | 8.63 | 0.83 | 8.80 | 6.60 | 9.90 | 0.643 |
| Phosphate | 12 | 3.69 | 1.19 | 3.55 | 2.20 | 6.00 | 9 | 3.96 | 1.53 | 3.60 | 2.90 | 7.80 | 0.910 |
| GOT | 15 | 50.33 | 95.24 | 25.00 | 10.00 | 392.00 | 10 | 47.70 | 36.12 | 36.50 | 14.00 | 129.00 | 0.246 |
| GPT | 28 | 27.11 | 44.14 | 20.00 | 7.00 | 249.00 | 19 | 27.05 | 16.91 | 25.00 | 5.00 | 60.00 | 0.151 |
Note:P value by Mann-Whitney U Test.
Abbreviations: AF, atrial fibrillation; BUN, blood urea nitrogen; eGFR, estimated glomerular filtration rate; GOT, glutamic oxaloacetic transaminase; GPT, glutamic pyruvic transaminase; HDL, high-density lipoprotein; LDL, low-density lipoprotein; Min, minimum; Max, maximum; SD, standard deviation.
Figure 2.
Change in estimated GFR between the two groups was measured over time (at months 3, 6, 12, and 18) after introduction of warfarin.
Notes: The Wilcoxon signed rank test was used to examine within-group improvement at different time points, showing an increase in the medians over time in group B. There was no significant difference in change in eGFR between the groups at any time point, but there was a trend towards improvement in renal function in group B.
Abbreviations: GFR, glomerular filtration rate; eGFR, estimated glomerular filtration rate.
Table 2.
Results of linear regression model using generalized estimating equation method for glomerular filtration rate
| Predictors | Estimate | SE | 95% CI | P value |
|---|---|---|---|---|
| (Intercept) | 118.504 | 13.758 | 91.539–145.469 | <0.001 |
| Group | ||||
| B | 6.066 | 2.355 | 1.450–10.683 | 0.010 |
| A | 0.000 | |||
| Stage | ||||
| 5 | −24.133 | 3.396 | −30.789–17.477 | <0.001 |
| 4 | −7.622 | 4.053 | −15.567–0.322 | 0.060 |
| 3 | 0.000 | |||
| Time (months) | ||||
| 18 | 2.057 | 4.484 | −6.730–10.845 | 0.646 |
| 15 | 3.210 | 3.359 | −3.374–9.795 | 0.339 |
| 12 | 5.241 | 3.703 | −2.017–12.499 | 0.157 |
| 9 | 3.400 | 3.471 | −3.402–10.203 | 0.327 |
| 6 | 3.657 | 3.091 | −2.401–9.716 | 0.237 |
| 3 | 5.046 | 3.798 | −2.398–12.490 | 0.184 |
| Baseline | 0.000 | |||
| LDL | −0.143 | 0.048 | −0.236–0.049 | 0.003 |
| CA | −8.662 | 1.689 | −11.973–5.350 | <0.001 |
Abbreviations: LDL, low density lipoprotein; CA, calcium; SE, standard error of the mean; CI, confidence interval.
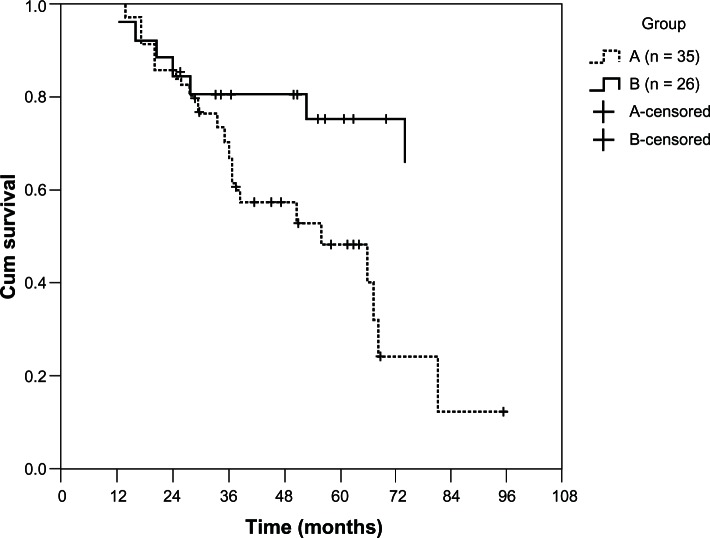
During the 12-year observation period, patients treated with warfarin had significantly (P = 0.013) better survival than group A (Figure 3). After multivariate Cox proportional hazards regression analysis (Table 3), the adjusted hazard ratio of mortality for group B patients was 0.318 (95% CI 0.120–0.846, P = 0.022). Using CKD stages or estimated GFR as the independent variable, we did not find a significant impact on patient survival. Age had an adverse effect on survival (hazard ratio 1.084, 95% CI 1.019–1.152, P = 0.011). Septic shock was the leading cause of death (n = 24, 60%), followed by cerebrovascular disease (n = 5, 12.5%), and cardiovascular disease (n = 5, 12.5%). Other causes of death were liver disease (n = 4, 10%) and sudden death (n = 2, 5%) of unknown cause. Two patients in each group suffered an ischemic stroke and two patients (one in group A and one in group B) had an intracranial hemorrhage.
Figure 3.
The survival curves for both groups were evaluated using the Kaplan–Meier method and the log rank test.
Notes: During the 12-year observational period, patients on warfarin had better survival than those not on warfarin (P = 0.013).
Table 3.
Multivariate Cox proportional hazards regression analysis of mortality
| β | SE | HR | 95% CI | P value | |
|---|---|---|---|---|---|
| Age at study enrollment | 0.066 | 0.029 | 1.068 | 1.009–1.131 | 0.023 |
| Estimated GFR | 0.008 | 0.019 | 1.008 | 0.971–1.047 | 0.675 |
| Group (B versus A) | −1.024 | 0.471 | 0.359 | 0.143–0.904 | 0.030 |
Abbreviations: CI, confidence interval; GFR, glomerular filtration rate; HR, adjusted hazard ratio; SE, standard error of the mean.
Discussion
The major benefit of warfarin is seen in those at highest risk of stroke, such as older people and those of any age with other risk factors, such as hypertension, left ventricular dysfunction, diabetes mellitus, or previous transient ischemic attack or stroke.20 Few studies have examined the effect of warfarin on renal function. After adjusting for related risk factors for renal dysfunction or renal function itself (CKD stages or estimated GFR), we found that warfarin therapy, when compared with an antiplatelet regimen, could slow the rate of deterioration of kidney function over 18 months and achieve longer survival in elderly patients with CKD and AF.
Warfarin appears to afford a long-term benefit on renal function in children with IgA nephropathy,21 although the mechanism is not understood. It has been shown that the binding of growth arrest-specific gene 6 to its cell surface receptor, Axl, stimulates mesangial cell proliferation and hypertrophy.22 Growth arrest-specific gene 6 is a vitamin K-dependent autocrine growth factor, and the growth arrest-specific gene 6/Axl pathway has been implicated in various kidney diseases, including diabetic nephropathy.23 The antiproliferative effect of warfarin, a vitamin K antagonist, is achieved at lower concentrations than its anticoagulant effect.24 Warfarin also inhibits vitamin K- dependent gamma carboxylase, increasing the intracellular glutamine level. The intracellular glutamine-glutamate cycle is the biosynthetic source of glutathione, a cellular antioxidant that prevents oxidative stress and modulates the apoptotic signaling pathway.25
The pathogenesis of warfarin-related nephropathy has been studied in the 5/6 nephrectomy animal model of CKD.26 Vitamin K-dependent proteins, matrix Gla protein and Gas6,27,28 inhibit vascular calcification and affect vascular smooth muscle cell migration and apoptosis. However, even if warfarin inhibits the activation of these two proteins and induces vascular calcification, the relationship between warfarin and warfarin-related nephropathy in humans has not yet been fully elucidated. A recent epidemiological study by Brodsky et al reported warfarin-related nephropathy in the presence of an INR > 3.0 in patients with and without CKD.16 Renal biopsy suggests that glomerular hemorrhage and subsequent renal tubular obstruction by red blood cell casts is a potential mechanism.29 In our study, the mean INR was 1.95 ± 1.01 and gross hematuria was not noted.
We found that warfarin maintaining a mean INR of 1.95 ± 1.01 significantly reduced all-cause mortality in elderly patients with AF and CKD compared with those treated with an antiplatelet regimen over 12 years, and there was no difference in the incidence of bleeding. Our results are comparable with those of the randomized controlled BAFTA (Birmingham Atrial Fibrillation Treatment of the Aged) trial.30 Ali et al also suggested that antiplatelet agents should not be an alternative means of anticoagulation in older people with AF due to lack of efficacy, and that bleeding risk should be assessed on an individual basis.31
Our study has some limitations. The study sample size was relatively small, the analysis was retrospective, the demographic studied was narrow, the results cannot be generalized, elderly patients with AF, CKD, and a high CHADS2 score also have other comorbidities (all of which have a negative impact on patients’ survival and renal function) and the study was conducted over 12 years. It might also be worth mentioning the possibility of chronology bias, in that survival may have been influenced by the introduction of lifestyle modification or other more effective drugs, except warfarin.
Conclusion
Warfarin therapy, as compared with an antiplatelet regimen, slowed the rate of decline in renal function over an 18-month period in elderly patients with AF and CKD and improved long-term survival.
Acknowledgments
The authors wish to thank Yi-Ping Sung for her kind help with data collection. We also thank CH Chang for revising the manuscript.
Footnotes
Disclosure
The authors report that they have no conflicts of interest in this work.
References
- 1.Feest TG, Rajamahesh J, Byrne C, et al. Trends in adult renal replacement therapy in the UK: 1982–2002. QJM. 2005;98(1):21–28. doi: 10.1093/qjmed/hci007. [DOI] [PubMed] [Google Scholar]
- 2.Lisowska A, Tycińska A, Knapp M, et al. The incidence and prognostic significance of cardiac arrhythmias and conduction abnormalities in patients with acute coronary syndromes and renal dysfunction. Kardiol Pol. 2011;69(12:):1242–1247. Polish. [PubMed] [Google Scholar]
- 3.Riley AB, Manning WJ. Atrial fibrillation: an epidemic in the elderly. Expert Rev Cardiovasc Ther. 2011;9(8):1081–1090. doi: 10.1586/erc.11.107. [DOI] [PubMed] [Google Scholar]
- 4.Nabauer M, Gerth A, Limbourg T, et al. The Registry of the German Competence NETwork on Atrial Fibrillation: patient characteristics and initial management. Europace. 2009;11(4):423–434. doi: 10.1093/europace/eun369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Furberg CD, Psaty BM, Manolio TA, et al. Prevalence of atrial fibrillation in elderly subjects (the Cardiovascular Health Study) Am J Cardiol. 1994;74(3):236–241. doi: 10.1016/0002-9149(94)90363-8. [DOI] [PubMed] [Google Scholar]
- 6.Horio T, Iwashima Y, Kamide K, et al. Chronic kidney disease as an independent risk factor for new-onset atrial fibrillation in hypertensive patients. J Hypertens. 2010;28(8):1738–1744. doi: 10.1097/HJH.0b013e32833a7dfe. [DOI] [PubMed] [Google Scholar]
- 7.Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285(18):2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 8.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham study. Stroke. 1991;22(8):983–988. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 9.Piccini JP, Wallace TW, Patel MR, Becker RC. Stroke prevention in atrial fibrillation. Cardiovasc Drugs Ther. 2011;25(6):561–570. doi: 10.1007/s10557-011-6334-4. [DOI] [PubMed] [Google Scholar]
- 10.Gage BF, Waterman AD, Shannon W, et al. Validation of clinical classification schemes for predicting stroke. Results from the national registry of atrial fibrillation. JAMA. 2001;285(22):2864–2870. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 11.Nakagawa K, Hirai T, Takashima S, et al. Chronic kidney disease and CHADS2 score independently predict cardiovascular events and mortality in patients with nonvalvular atrial fibrillation. Am J Cardiol. 2011;107(6):912–916. doi: 10.1016/j.amjcard.2010.10.074. [DOI] [PubMed] [Google Scholar]
- 12.Sandhu RK, Kurth T, Conen D, et al. Relation of renal function to risk for incident atrial fibrillation in Women. Am J Cardiol. 2012;109(4):538–542. doi: 10.1016/j.amjcard.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hart RG, Pearce LA, Asinger RW, et al. Warfarin in atrial fibrillation patients with moderate chronic kidney disease. Clin J Am Soc Nephrol. 2011;6(11):2599–2604. doi: 10.2215/CJN.02400311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials. Arch Intern Med. 1994;154(13):1449–1457. [No authors listed] [PubMed] [Google Scholar]
- 15.Tulner LR, Van Campen JP, Kuper IM, et al. Reasons for under treatment with oral anticoagulants in frail geriatric outpatients with atrial fibrillation: a prospective, descriptive study. Drugs Aging. 2010;27(1):39–50. doi: 10.2165/11319540-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 16.Brodsky SV, Nadasdy T, Rovin BH, et al. Warfarin-related nephropathy occurs in patients with and without chronic kidney disease and is associated with an increased mortality rate. Kidney Int. 2011;80(2):181–189. doi: 10.1038/ki.2011.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levey AS, Coresh J, Balk E, et al. National Kidney Foundation National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139(2):137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 18.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, STROBE Initiative The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med. 2007;4(10):e296. doi: 10.1371/journal.pmed.0040296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Camm AJ, Kirchhof P, Lip GY, et al. European Heart Rhythm Association, European Association for Cardio-Thoracic Surgery, Guidelines for the management of atrial fibrillation. Eur Heart J. 2010;31(19):2369–2429. doi: 10.1093/eurheartj/ehq278. [DOI] [PubMed] [Google Scholar]
- 20.Ezekowitz MD, Levine JA. Preventing stroke in patients with atrial fibrillation. JAMA. 1999;281(19):1830–1835. doi: 10.1001/jama.281.19.1830. [DOI] [PubMed] [Google Scholar]
- 21.Kamei K, Nakanishi K, Ito S, et al. Long-term results of a randomized controlled trial in childhood IgA nephropathy. Clin J Am Soc Nephrol. 2011;6(6):1301–1307. doi: 10.2215/CJN.08630910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yanagita M. The role of the vitamin K-dependent growth factor Gas6 in glomerular pathophysiology. Curr Opin Nephrol Hypertens. 2004;13(4):465–470. doi: 10.1097/01.mnh.0000133981.63053.e9. [DOI] [PubMed] [Google Scholar]
- 23.Nagai K, Matsubara T, Mima A, et al. Gas6 induces Akt/mTOR-mediated mesangial hypertrophy in diabetic nephropathy. Kidney Int. 2005;68(2):552–561. doi: 10.1111/j.1523-1755.2005.00433.x. [DOI] [PubMed] [Google Scholar]
- 24.Yanagita M, Ishii K, Ozaki H, et al. Mechanism of inhibitory effect of warfarin on mesangial cell proliferation. J Am Soc Nephrol. 1999;10(12):2503–2509. doi: 10.1681/ASN.V10122503. [DOI] [PubMed] [Google Scholar]
- 25.Matés JM, Pérez-Gómez C, Núñez de Castro I, et al. Glutamine and its relationship with intracellular redox status, oxidative stress and cell proliferation/death. Int J Biochem Cell Biol. 2002;34(5):439–458. doi: 10.1016/s1357-2725(01)00143-1. [DOI] [PubMed] [Google Scholar]
- 26.Ozcan A, Ware K, Calomeni E, et al. 5/6 Nephrectomy as a validated rat model mimicking human warfarin-related nephropathy. Am J Nephrol. 2012;35(4):356–364. doi: 10.1159/000337918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zebboudj AF, Shin V, Bostrom K. Matrix GLA protein and BMP-2 regulate osteoinduction in calcifying vascular cells. J Cell Biochem. 2003;90(4):756–765. doi: 10.1002/jcb.10669. [DOI] [PubMed] [Google Scholar]
- 28.Proudfoot D, Skepper JN, Hegyi L, et al. Apoptosis regulates human vascular calcification in vitro: evidence for initiation of vascular calcification by apoptotic bodies. Circ Res. 2000;87(11):1055–1062. doi: 10.1161/01.res.87.11.1055. [DOI] [PubMed] [Google Scholar]
- 29.Brodsky SV, Satoskar A, Chen J, et al. Acute kidney injury during warfarin therapy associated with obstructive tubular red blood cell casts: a report of 9 cases. Am J Kidney Dis. 2009;54(6):1121–1126. doi: 10.1053/j.ajkd.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 30.Mant J, Hobbs FD, Fletcher K, et al. Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study, BAFTA): a randomized controlled trial. Lancet. 2007;370(9586):493–503. doi: 10.1016/S0140-6736(07)61233-1. [DOI] [PubMed] [Google Scholar]
- 31.Ali A, Bailey C, Abdelhafiz AH. Stroke prevention with oral anticoagulation in older people with atrial fibrillation – a pragmatic approach. Aging Dis. 2012;3(4):339–351. [PMC free article] [PubMed] [Google Scholar]