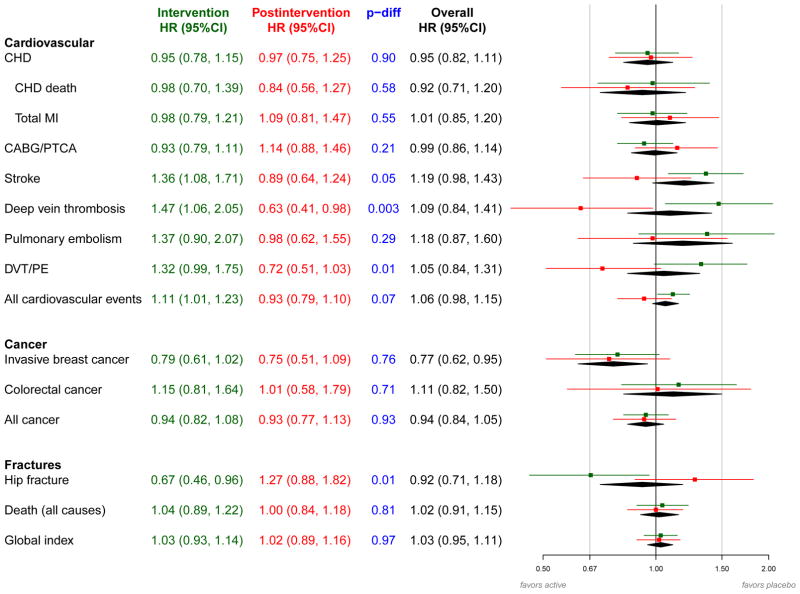
Figure 2.
Effects of randomized assignment to conjugated equine estrogen vs. placebo on clinical outcomes during intervention phase and post-intervention in the Women’s Health Initiative Estrogen-alone Trial (details in Appendix 2 Table).
aHazard ratios and 95% confidence intervals are shown for the intervention phase in green, the post-intervention period in red, and the overall period in black. The P-difference (shown in blue) tests whether the hazard ratio for the intervention phase equals the hazard ratio for the post-intervention period.
bHazard ratios are derived from proportional hazards models stratified by prior disease (for outcomes where women were eligible for enrollment with and without the prevalent condition), age and dietary modification randomization group. Models for the overall 10.7 mean year follow-up period include a time dependent term for trial phase. For the intervention and overall phases, time to event equals 0 on date of randomization. For the postintervention phase, time to event equals 0 on February 29, 2004.
Abbreviations: CABG, coronary artery bypass graft; CEE, conjugated equine estrogen; CHD, coronary heart disease; CI, confidence interval; CVD, cardiovascular disease; DVT, deep vein thrombosis; HR, hazard ratio; MI, myocardial infarction; PE, pulmonary embolism; PTCA, percutaneous transluminal coronary angioplasty.