Figure 3.
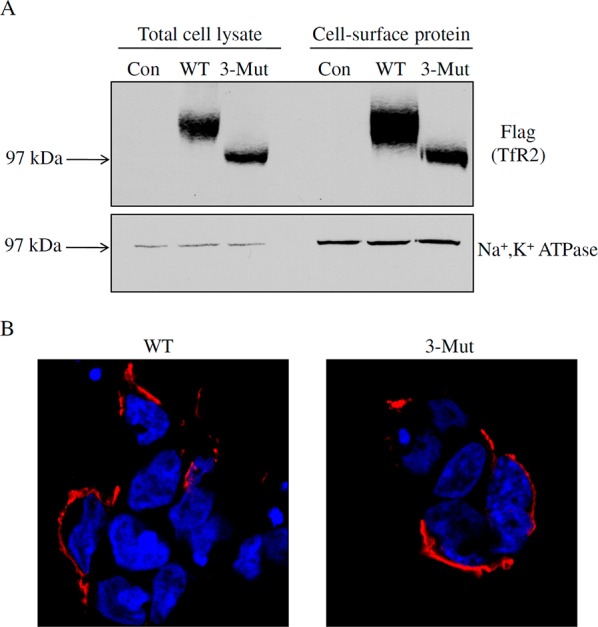
N-Linked glycosylation does not affect plasma membrane localization of hTfR2. (A) HEK 293 cells were transiently transfected with empty pcDNA3 vector (Con), wild-type hTfR2-FLAG (WT), or its mutant with all three glycosylated Asn residues replaced with alanines (3-Mut). After 24 h, total cell lysates were harvested by using NETT cell lysis buffer, while cell surface proteins were labeled with cell membrane-impermeable NHS-SS-biotin. Samples were analyzed by Western blotting for TfR2. After being stripped, blots were reprobed for Na+,K+-ATPase as a marker for plasma membrane proteins. (B) For immunofluorescence, HEK 293 cells were transiently transfected with WT or 3-Mut hTfR2-FLAG. Twenty-four hours after transfection, WT or 3-Mut TfR2 was detected by using mouse anti-TfR2 antibody followed by Alexa Fluor-594-conjugated secondary antibody. Images show that both WT hTfR2 and the nonglycosylated form of hTfR2 (3-Mut) were detected at the plasma membrane. DAPI was used to stain nuclei. Data are representative of one of three independent experiments.
