Table 1. Optimization of Reaction Conditionsa.
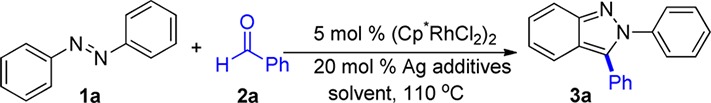
| entry | solvent | Ag salt | yield (external)b |
|---|---|---|---|
| 1 | DCE | AgSbF6 | 46 |
| 2 | DCE | none | tracec |
| 3 | THF | AgSbF6 | 46 |
| 4 | HOAc | AgSbF6 | 64 |
| 5 | dioxane | AgSbF6 | 81 |
| 6 | dioxane | AgBF4 | 52 |
| 7 | dioxane | AgPF6 | 15 |
| 8 | dioxane | AgBC24F20 | 40 |
| 9 | dioxane | none | 0 |
| 10 | dioxane | AgSbF6 | 0d |
Conditions: 1 (0.10 mmol), 2 (0.20 mmol) in 0.5 mL of solvent for 24 h.
Determined by 1H NMR relative to 2,6-dimethoxytoluene as external standard.
In place of (Cp*RhCl2)2, Cp*Rh(CH3CN)3(SbF6)2 was used.
No (Cp*RhCl2)2 was added.
