Abstract
Cell-division-cycle protein 48 (CDC48) is an essential, conserved ATP-driven chaperone in eukaryotic cells, which functions in diverse cellular processes including the targeting of misfolded and aggregated proteins for degradation via proteasomal and aggresomal-autophagic pathways. We recently demonstrated that plant CDC48 localizes to and interacts with Tobacco mosaic virus (TMV) movement protein (MP) in ER-associated viral protein inclusions. Our data suggest that CDC48 participates in the clearance of these viral protein inclusions in an ER-assisted protein degradation (ERAD)-like mechanism. As TMV MP-inclusions formed at late infection stages resemble aggresomes, we here propose that TMV MP enters both, ERAD-like and aggresomal pathways in its host cells and that CDC48 coordinates these processes. Moreover, as viruses often exploit host pathways for replication and spread, we propose a model in which CDC48 functions in the degradation pathway of overaccumulating viral protein and also actively participates in the regulation of TMV replication and cell-to-cell movement.
Keywords: Tobacco mosaic virus, ER-assisted protein degradation, aggresome, endoplasmic reticulum, microtubules, protein inclusion, replication complex, virus infection
TMV is an RNA virus that replicates in association with ER membranes.1,2 The MP of this virus is required for intercellular spread of the viral RNA (vRNA) through plasmodesmata (PD) and localizes to PD and small mobile ER-associated particles at the cell cortex during early infection and to aggresome-like, ER-associated inclusions and microtubules during late infection.2-5 We recently provided evidence for CDC48-mediated extraction of MP from ER-inclusions into the cytosol leading to accumulation of the protein along microtubules and to degradation of MP.6 We suggested that this process represents primarily a defense response of the plant to remove MP from the ER-transport pathway. However, CDC48-mediated extraction of MP from inclusions may also represent a viral strategy to switch functions of multifunctional viral proteins. CDC48-mediated retrotranslocation of mammalian retroviral Rem protein from the ER as a prerequisite for expression and nuclear export of viral RNA, for example, has been previously reported.7
As CDC48 has been shown to function in proteasomal and aggresomal-autophagic pathways,8,9 and protein inclusions formed at late stages of TMV infection resemble aggesomes,2 we here propose that TMV MP enters both, ERAD-like and aggresomal pathways upon infection. We discuss possible advantages of inclusion formation and microtubule association of TMV MP during infection and propose a model in which CDC48 function in the retrotranslocation of MP from viral protein inclusions contributes to the regulation of TMV replication and cell-to-cell movement.
Plant Viral Protein Inclusions Resemble Aggresomes and May Compartmentalize Viral Replication
It has been suggested that viral protein inclusions are reminiscent of aggresomes and serve as platforms for the generation of viral replication sites.10-12 Aggresomes are perinuclear deposits for terminally misfolded proteins in mammalian cells, which are eventually cleared by autophagy.13 Such specialized compartments formed by viral proteins during infection could protect the host from possible cytotoxic effects of viral proteins. That aggresomes have a cytoprotective role has been demonstrated in yeast.14 In addition, viral protein inclusions could increase viral replication efficiency by concentrating viral components and by facilitating the recruitment of host factors. Moreover, such compartments could shield viral RNA and proteins from recognition by host immune responses such as RNA silencing or receptor-mediated defense. Indeed, the ER-associated, aggresome-like inclusions formed at late stages of TMV infection harbor viral replication complexes (VRCs),1,2 thus suggesting a role of TMV inclusions in compartmentalization of viral replication. Also the highly organized structure of inclusions formed by triple gene block protein 1 during Potato virus X infection suggests a role of these structures in the compartmentalization of the virus replication cycle leading to increased production of progeny virus.15 Moreover, aggresome-like inclusions formed during Cauliflower mosaic virus infection were shown to function in facilitating vector transmission of the virus.16,17 Thus, it appears likely that plant viral protein inclusions serve a function beneficial for infection.
The Formation of Plant Viral Protein Inclusions Depends on Microtubules and Shares Similarities with the Formation of Aggresomes
In mammalian and yeast cells the transport of proteins to the aggresome is dependent on microtubules and tubulin-associated factors. Notably, the CDC48-interacting, tubulin-associated histone deacetylase 6 and microtubules are involved in gathering and transport of aggregated proteins to the aggresome located at the microtubule organizing center (MTOC).13,18,19
There is some evidence indicating that microtubules are also involved in the dissolution and formation of plant viral protein inclusions. For example, Cauliflower mosaic virus P2 and P3 appear to be exported from multiple, electron dense inclusions to microtubules during infection, and intact microtubules are important for subsequent formation of an electron lucent, aggresome-like transmission body containing P2 and P3.16 In addition, the formation of aggresome-like structures formed by Potato leafroll virus MP upon inhibition of the 26S proteasome appeared to depend on intact microtubules.20 During TMV infection small, mobile, ER-associated, microtubule proximal MP complexes presumably containing vRNA21 are formed early after infection, fuse into larger inclusions as infection progresses and become immobile when MP starts to accumulate along microtubules.3 As no single microtubule organizing center exists in plants, and microtubules nucleate at existing microtubules22 anchoring sites of MP inclusions might represent sites of microtubule nucleation, and correspond to the MTOC anchoring the aggresome in mammalian cells. Consistently, MP interacted with microtubule nucleation sites in mammalian cells,23,24 and with the microtubule assembly factors EB1:GFP25 and γ-tubulin21 in plants. Thus, in analogy to the formation of aggresomes in mammalian cells, transport of plant viral protein for the formation of aggresome-like inclusions appears to involve microtubules.
CDC48 as a Regulator of TMV Infection
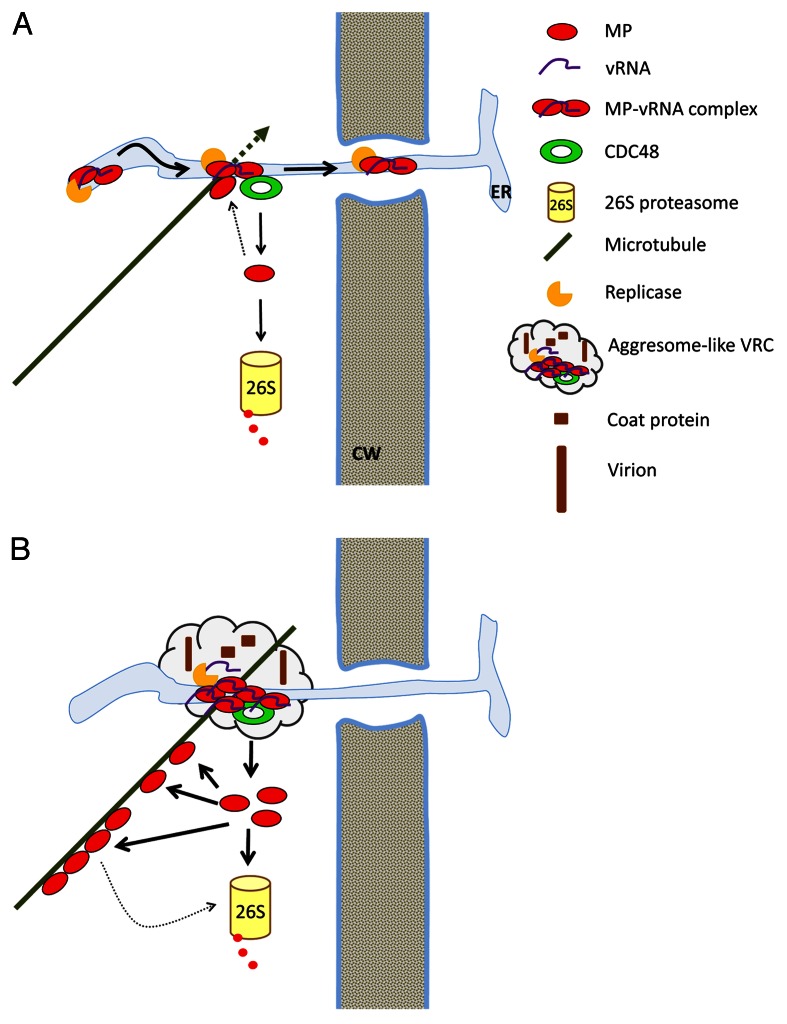
We discussed above that many plant viral proteins form inclusions resembling aggresomes upon infection and that microtubule dynamics appears to be important for intracellular transport of viral protein and the formation of aggresome-like inclusions. Moreover, CDC48 has been proposed to mediate the delivery of proteins to the proteasome or the aggresome, respectively, depending on the proteostatic state of the cell.26 As a platform for substrate binding and substrate processing cofactors such as ubiquitin E3 ligases and deubiquitinating enzymes,9,27 it is well possible that CDC48 and its cofactors dictate proteins into distinct degradation pathways. Based on our recent finding that CDC48 extracts the TMV MP from ER-associated inclusions into the cytosol to promote MP degradation as well as association of MP with microtubules, where it interferes with microtubule dynamics, we here propose a model for CDC48 function in the regulation of TMV infection (Fig. 1).
Figure 1. Model for a role of CDC48 in the regulation of TMV replication and cell-to-cell movement. (A) At early infection stages, the equilibrium between ER-associated and microtubule-associated MP supports virus movement. (B) At late infection stages, CDC48 function interferes with further virus movement and supports the massive production of progeny virus. ER, endoplasmic reticulum; CW, cell wall.
Early after infection of a new cell MP-vRNA complexes possibly also containing replicase diffuse along the ER membrane toward PD1,2,4 (Fig. 1A). MP-vRNA complexes anchor at ER-microtubule attachment sites, where misfolded MP is extracted by CDC48 for degradation by the 26S proteasome.6,28 At early infection stages, only little MP is present and only little MP is misfolded and extracted. Microtubule polymerization leads to release of MP-vRNA complexes from these attachment sites and resumed diffusion along the ER. Some of these MP-vRNA complexes eventually arrive at PD and move to neighboring cells.29 Thus, at early infection stages, the equilibrium between ER-associated and microtubule-associated MP supports virus movement.
At later infection stages, more misfolded MP is extracted from MP-complexes, the 26S proteasome becomes saturated and increased amounts of MP accumulate along microtubules. Stabilization of microtubules by MP immobilizes the MP-vRNA complexes, hinders further transport of the MP-vRNA complexes along the ER and into neighboring cells and forms a scaffold for further accumulation of MP, vRNA and replicase at microtubule-anchored sites. This process leads to the formation of large, aggresome-like, ER-associated inclusions, the late VRCs (Fig. 1B). Extraction of MP from ER-inclusions by CDC48 may render the RNA translatable and consequently assist further replication of the virus. Consistent with this hypothesis, it has been shown that TMV vRNA is untranslatable in MP-vRNA complexes in vitro, and that translatability depends on the affinity of the MP to the RNA.30,31 Moreover, it has been shown that CDC48 genetically interacts with ribonucleoproteins involved in translational regulation in Drosophila.32 In agreement with our hypothesis that CDC48 removes MP from vRNA during infection, the authors suggested that CDC48 extracts proteins from these ribonucleoprotein complexes.32
In conclusion, while CDC48 function may represent a host defense response to remove MP from the ER-transport pathway by recognizing the protein as misfolded and targeting it for degradation in an ERAD-like pathway, it may, at the same time, be exploited by the virus to increase replication efficiency, and to limit virus movement behind the infection front. In future work, it will be interesting to test whether CDC48 function is indeed required for the switch between the transport and replication form of the virus during infection.
Acknowledgments
Funding was provided by the Swiss National Science Foundation and by a Zurich-Basel Plant Science Center-Syngenta fellowship.
Glossary
Abbreviations:
- CDC48
cell-division-cycle protein 48
- ERAD
ER-assisted protein degradation
- MP
movement protein
- MTOC
microtubule organizing center
- PD
plasmodesmata
- TMV
Tobacco mosaic virus
- VRC
viral replication complex
- vRNA
viral RNA
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/22865
References
- 1.Heinlein M, Padgett HS, Gens JS, Pickard BG, Casper SJ, Epel BL, et al. Changing patterns of localization of the tobacco mosaic virus movement protein and replicase to the endoplasmic reticulum and microtubules during infection. Plant Cell. 1998;10:1107–20. doi: 10.1105/tpc.10.7.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Más P, Beachy RN. Replication of tobacco mosaic virus on endoplasmic reticulum and role of the cytoskeleton and virus movement protein in intracellular distribution of viral RNA. J Cell Biol. 1999;147:945–58. doi: 10.1083/jcb.147.5.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyko V, Hu Q, Seemanpillai M, Ashby J, Heinlein M. Validation of microtubule-associated Tobacco mosaic virus RNA movement and involvement of microtubule-aligned particle trafficking. Plant J. 2007;51:589–603. doi: 10.1111/j.1365-313X.2007.03163.x. [DOI] [PubMed] [Google Scholar]
- 4.Niehl A, Heinlein M. Cellular pathways for viral transport through plasmodesmata. Protoplasma. 2011;248:75–99. doi: 10.1007/s00709-010-0246-1. [DOI] [PubMed] [Google Scholar]
- 5.Peña EJ, Heinlein M. RNA transport during TMV cell-to-cell movement. Front Plant Sci 2012; 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Niehl A, Amari K, Gereige D, Brandner K, Mély Y, Heinlein M. Control of Tobacco mosaic virus movement protein fate by CELL-DIVISION-CYCLE protein 48 (CDC48) Plant Physiol. 2012 doi: 10.1104/pp.112.207399. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Byun H, Halani N, Mertz JA, Ali AF, Lozano MM, Dudley JP. Retroviral Rem protein requires processing by signal peptidase and retrotranslocation for nuclear function. Proc Natl Acad Sci U S A. 2010;107:12287–92. doi: 10.1073/pnas.1004303107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ju J-S, Weihl CC. p97/VCP at the intersection of the autophagy and the ubiquitin proteasome system. Autophagy. 2010;6:283–5. doi: 10.4161/auto.6.2.11063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meyer H, Bug M, Bremer S. Emerging functions of the VCP/p97 AAA-ATPase in the ubiquitin system. Nat Cell Biol. 2012;14:117–23. doi: 10.1038/ncb2407. [DOI] [PubMed] [Google Scholar]
- 10.Jackson WT, Giddings TH, Jr., Taylor MP, Mulinyawe S, Rabinovitch M, Kopito RR, et al. Subversion of cellular autophagosomal machinery by RNA viruses. PLoS Biol. 2005;3:e156. doi: 10.1371/journal.pbio.0030156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laliberté J-F, Sanfaçon H. Cellular remodeling during plant virus infection. Annu Rev Phytopathol. 2010;48:69–91. doi: 10.1146/annurev-phyto-073009-114239. [DOI] [PubMed] [Google Scholar]
- 12.Wileman T. Aggresomes and autophagy generate sites for virus replication. Science. 2006;312:875–8. doi: 10.1126/science.1126766. [DOI] [PubMed] [Google Scholar]
- 13.Chin LS, Olzmann JA, Li L. Parkin-mediated ubiquitin signalling in aggresome formation and autophagy. Biochem Soc Trans. 2010;38:144–9. doi: 10.1042/BST0380144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y, Meriin AB, Zaarur N, Romanova NV, Chernoff YO, Costello CE, et al. Abnormal proteins can form aggresome in yeast: aggresome-targeting signals and components of the machinery. FASEB J. 2009;23:451–63. doi: 10.1096/fj.08-117614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tilsner J, Linnik O, Wright KM, Bell K, Roberts AG, Lacomme C, et al. The TGB1 movement protein of Potato virus X reorganizes actin and endomembranes into the X-body, a viral replication factory. Plant Physiol. 2012;158:1359–70. doi: 10.1104/pp.111.189605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martinière A, Gargani D, Uzest M, Lautredou N, Blanc S, Drucker M. A role for plant microtubules in the formation of transmission-specific inclusion bodies of Cauliflower mosaic virus. Plant J. 2009;58:135–46. doi: 10.1111/j.1365-313X.2008.03768.x. [DOI] [PubMed] [Google Scholar]
- 17.Khelifa M, Journou S, Krishnan K, Gargani D, Espérandieu P, Blanc S, et al. Electron-lucent inclusion bodies are structures specialized for aphid transmission of cauliflower mosaic virus. J Gen Virol. 2007;88:2872–80. doi: 10.1099/vir.0.83009-0. [DOI] [PubMed] [Google Scholar]
- 18.Kawaguchi Y, Kovacs JJ, McLaurin A, Vance JM, Ito A, Yao T-P. The deacetylase HDAC6 regulates aggresome formation and cell viability in response to misfolded protein stress. Cell. 2003;115:727–38. doi: 10.1016/S0092-8674(03)00939-5. [DOI] [PubMed] [Google Scholar]
- 19.Ouyang H, Ali YO, Ravichandran M, Dong A, Qiu W, MacKenzie F, et al. Protein aggregates are recruited to aggresome by histone deacetylase 6 via unanchored ubiquitin C termini. J Biol Chem. 2012;287:2317–27. doi: 10.1074/jbc.M111.273730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vogel F, Hofius D, Sonnewald U. Intracellular trafficking of Potato leafroll virus movement protein in transgenic Arabidopsis. Traffic. 2007;8:1205–14. doi: 10.1111/j.1600-0854.2007.00608.x. [DOI] [PubMed] [Google Scholar]
- 21.Sambade A, Brandner K, Hofmann C, Seemanpillai M, Mutterer J, Heinlein M. Transport of TMV movement protein particles associated with the targeting of RNA to plasmodesmata. Traffic. 2008;9:2073–88. doi: 10.1111/j.1600-0854.2008.00824.x. [DOI] [PubMed] [Google Scholar]
- 22.Murata T, Hasebe M. Microtubule-dependent microtubule nucleation in plant cells. J Plant Res. 2007;120:73–8. doi: 10.1007/s10265-006-0054-z. [DOI] [PubMed] [Google Scholar]
- 23.Boyko V, Ferralli J, Ashby J, Schellenbaum P, Heinlein M. Function of microtubules in intercellular transport of plant virus RNA. Nat Cell Biol. 2000;2:826–32. doi: 10.1038/35041072. [DOI] [PubMed] [Google Scholar]
- 24.Ferralli J, Ashby J, Fasler M, Boyko V, Heinlein M. Disruption of microtubule organization and centrosome function by expression of tobacco mosaic virus movement protein. J Virol. 2006;80:5807–21. doi: 10.1128/JVI.00254-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brandner K, Sambade A, Boutant E, Didier P, Mély Y, Ritzenthaler C, et al. Tobacco mosaic virus movement protein interacts with green fluorescent protein-tagged microtubule end-binding protein 1. Plant Physiol. 2008;147:611–23. doi: 10.1104/pp.108.117481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ju J-S, Weihl CC. Inclusion body myopathy, Paget’s disease of the bone and fronto-temporal dementia: a disorder of autophagy. Hum Mol Genet. 2010;19(R1):R38–45. doi: 10.1093/hmg/ddq157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stolz A, Hilt W, Buchberger A, Wolf DH. Cdc48: a power machine in protein degradation. Trends Biochem Sci. 2011;36:515–23. doi: 10.1016/j.tibs.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 28.Reichel C, Beachy RN. Degradation of tobacco mosaic virus movement protein by the 26S proteasome. J Virol. 2000;74:3330–7. doi: 10.1128/JVI.74.7.3330-3337.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kawakami S, Watanabe Y, Beachy RN. Tobacco mosaic virus infection spreads cell to cell as intact replication complexes. Proc Natl Acad Sci U S A. 2004;101:6291–6. doi: 10.1073/pnas.0401221101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karpova OV, Ivanov KI, Rodionova NP, Dorokhov YuL, Atabekov JG. Nontranslatability and dissimilar behavior in plants and protoplasts of viral RNA and movement protein complexes formed in vitro. Virology. 1997;230:11–21. doi: 10.1006/viro.1997.8472. [DOI] [PubMed] [Google Scholar]
- 31.Karpova OV, Rodionova NP, Ivanov KI, Kozlovsky SV, Dorokhov YL, Atabekov JG. Phosphorylation of tobacco mosaic virus movement protein abolishes its translation repressing ability. Virology. 1999;261:20–4. doi: 10.1006/viro.1999.9842. [DOI] [PubMed] [Google Scholar]
- 32.Ritson GP, Custer SK, Freibaum BD, Guinto JB, Geffel D, Moore J, et al. TDP-43 mediates degeneration in a novel Drosophila model of disease caused by mutations in VCP/p97. J Neurosci. 2010;30:7729–39. doi: 10.1523/JNEUROSCI.5894-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]