Figure 7.
Silencing of c-Abl Leads to Impaired Mammary Lumen Formation and Branching Morphogenesis
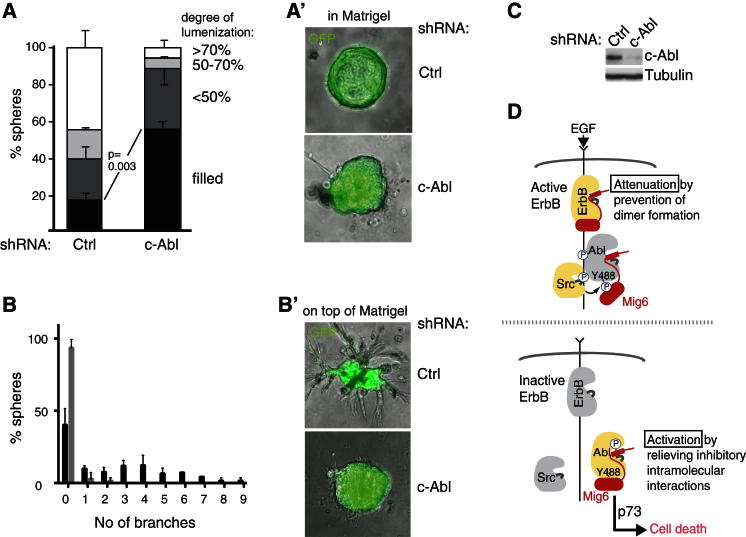
(A–C) pMECs were subjected to lentivirus-mediated shRNA silencing of c-Abl and cultured inside (A and A′) or on top of (B and B′) Matrigel. (A) The degree of luminal filling of individual acini was determined in a blind fashion from stacks of phase contrast and GFP fluorescence images (see A′ for representative acini). The proportion of spheres within the indicated subcategories are represented (n = 3 independent experiments with a total of 120 control and 118 Abl knockdown (k.d.) acini analyzed). (B) The number of branches of individual spheres cultured on top of Matrigel are plotted, n = 3 independent experiments with a total of 83 (ctrl) and 72 (Abl k.d.) acini analyzed. p = 0.006 for difference in mean number of branches per ctrl versus acini. (B′) Phase contrast/GFP images of representative acini. (C) Western analysis for indicated proteins of pMECs infected with the nontargeting or c-Abl-targeting shRNAs, confirming efficient knockdown. p values were determined by two-tailed unpaired Student's t test (A) or Mann-Whitney test (B), while error bars indicate SEM.
(D) Model illustrating the molecular switch mechanism by which Mig6 senses ErbB receptor inactivation. In the presence of EGF the ErbB-binding region of Mig6 (red) binds to the active ErbB receptors interfering with kinase domain dimer formation (Zhang et al., 2007a). C-Src is recruited to and activated by active ErbB receptors and phosphorylates c-Abl on Y488, thereby preventing Mig6 from activating c-Abl to induce p73-dependent cell death. Upon ErbB receptor inactivation c-Abl and Mig6 dissociate from the receptor, leading to de-phosphorylation of c-Abl on Y488, which enable Mig6 to activate c-Abl to trigger p73-dependent cell death.