Figure 4.
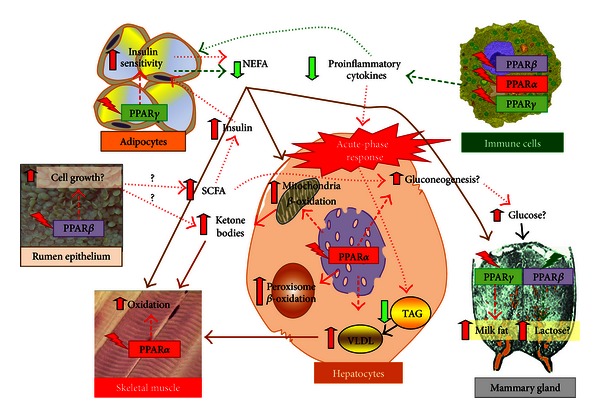
Improving transition from pregnancy into lactation in high producing dairy cows by nutrigenomics approach through PPAR isotypes: a hypothesis. The liver buffer cells from the excessive concentration of circulating nonesterified fatty acids (NEFA) by both catabolizing long-chain fatty acids (LCFA) with production of ketone bodies (KB) and esterifying them as triacylglycerol (TAG). The TAG are then accumulated in lipid droplets and packed into VLDL for release into the bloodstream. The liver is also induced by proinflammatory cytokines to produce positive acute phase proteins (+APP) taking away hepatic resources for normal liver functions. Despite the decrease in peripheral insulin concentration postpartum, the activation of PPARγ prior to parturition can decrease NEFA postpartum through greater insulin sensitivity primarily on the adipose tissue. The activation of PPARβ/δ (PPARβ in the figure) via LCFA can increase rumen epithelium growth with consequent larger production of short-chain fatty acids (SCFA) including propionate, which stimulate insulin production, and butyrate, augmenting the KB in blood. The increased activation of PPARα just before parturition and during the first 14 days postpartum in the liver and muscle can increase NEFA oxidation with greater proportion of KB produced per amount of NEFA uptake. The activation of PPARα in the liver has the potential to increase gluconeogenesis and VLDL synthesis. The KB can serve as fuels by skeletal muscle instead of NEFA and glucose; both molecules are substrates for mammary gland. In this tissue, the activation of PPARγ postpartum should increase or maintain milk fat. In addition, the inhibition of PPARβ/δ postpartum can potentially increase glucose import with a consequent increase in lactose synthesis, and hence, milk yield. The activation of PPAR isotypes just prior to parturition and during the first two weeks post-partum should diminish the inflammatory-like conditions preventing, on one hand, the stimulation of NEFA release and, on the other hand, hepatic acute-phase reaction, both determined by proinflammatory cytokines. This coordinated set of reactions should provide an ideal metabolic situation leading to a smoother transition from pregnancy into lactation, that is, allow the liver to allocate its resources for “normal” functions. As a consequence of this, the incidence of diseases typical of the peripartal period would be reduced, and hence, cows with higher performance and more healthy. Regular dashed arrows represent “effect on” due to PPAR isotype activation/inhibition, and round dot arrows denote secondary (or indirect) effects of PPAR isotype activation. In both cases red = activation or increase and green = inhibition or decrease.
