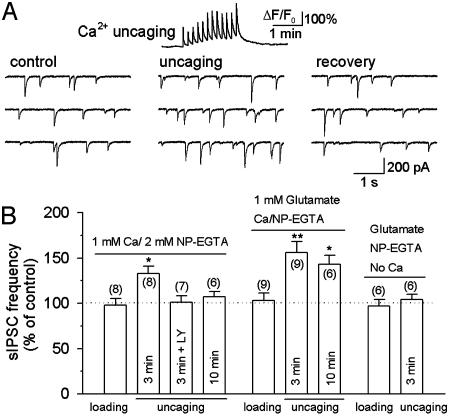
Fig. 5.
Modulation of sIPSCs by uncaging NP-EGTA loaded through whole-cell recording. (A Upper) A representative trace showing the time course of Ca2+ transients (ΔF/F0) induced by Ca2+ uncaging. Ca2+/NP-EGTA (1 mM/2 mM) was loaded into the astrocyte for 3 min before uncaging. (A Lower) Ca2+ uncaging produced a reversible increase in the frequency of sIPSCs in interneurons. (B) Pooled data show the effect of NP-EGTA loading, uncaging, and glutamate infusion on the frequency of sIPSCs in interneurons. Whole-cell loading of glutamate and/or NP-EGTA had no effect on sIPSCs. After loading 1 mM Ca2+/2 mM NP-EGTA for 3 min in astrocytes, Ca2+ uncaging produced a significant increase in the frequency of sIPSCs, which was blocked by the GluR5-containing kainate receptor antagonist LY293558 (LY, 30 μM). After loading Ca2+/NP-EGTA for 10 min, Ca2+ uncaging had no significant effect on sIPSCs (Left). The time-dependent rundown of the responses was mainly due to the washout of glutamate in whole-cell recording, because including 1 mM glutamate in the recording solution significantly retarded the rundown (Center). Uncaging NP-EGTA alone (in the absence of added Ca2+) had no effect on sIPSCs (Right). The number of cells tested in each group is shown in parentheses. *, P < 0.05; **, P < 0.01 compared with control or loading groups by ANOVA with Dunnett's test.