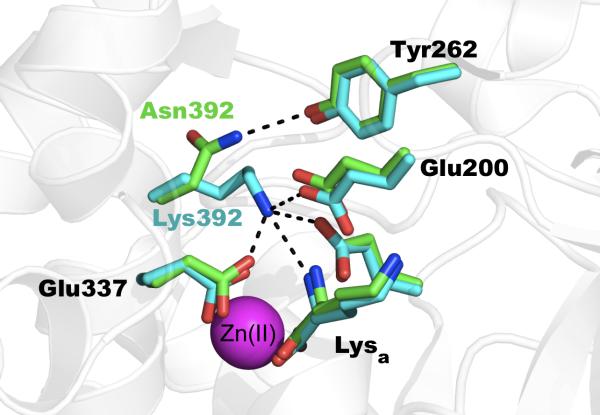
Figure 5.
Atomic interactions between the side-chain of the polymorphic position 392 in ERAP2. Asn 392 makes hydrogen bonding interactions with Tyr262. Lys392, approaches the N-terminus of the active-site bound lysine amino acid and makes several salt-bridge interactions with catalytically important Glu residues.