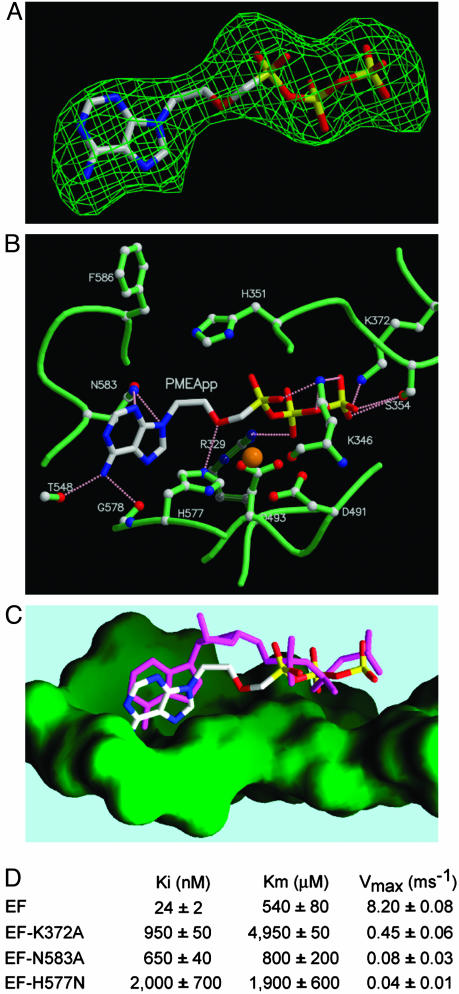
Fig. 4.
Structural analyses of the interactions of PMEApp with EF. (A) Omit map of PMEApp in the catalytic site of EF. The model of PMEApp is shown with the 3σ cutoff electron density map. The color of PMEApp is kept the same throughout, in which carbon, oxygen, nitrogen, and phosphorus are white, red, blue, and yellow, respectively. (B) The interactions of the EF-CaM complex with PMEApp. The structure of EF-CaM in the presence of PMEApp is in green, and the atoms of carbon, oxygen, and nitrogen are white, red, and blue, respectively. A catalytic metal, ytterbium, is orange, and hydrogen bonds are represented in pink. (C) Comparison of PMEApp with 3′-deoxy-ATP. The molecular surface of the ventral side of the active site is green. 3′-Deoxy-ATP is in magenta. (D) Kinetic analysis of wild-type and mutant forms of EF, EF3-K372A, EF3-N583A, and EF3-H577N. Representative kinetic data for D are supplied in Fig. 8, which is published as supporting information on the PNAS web site.