Fig. 1.
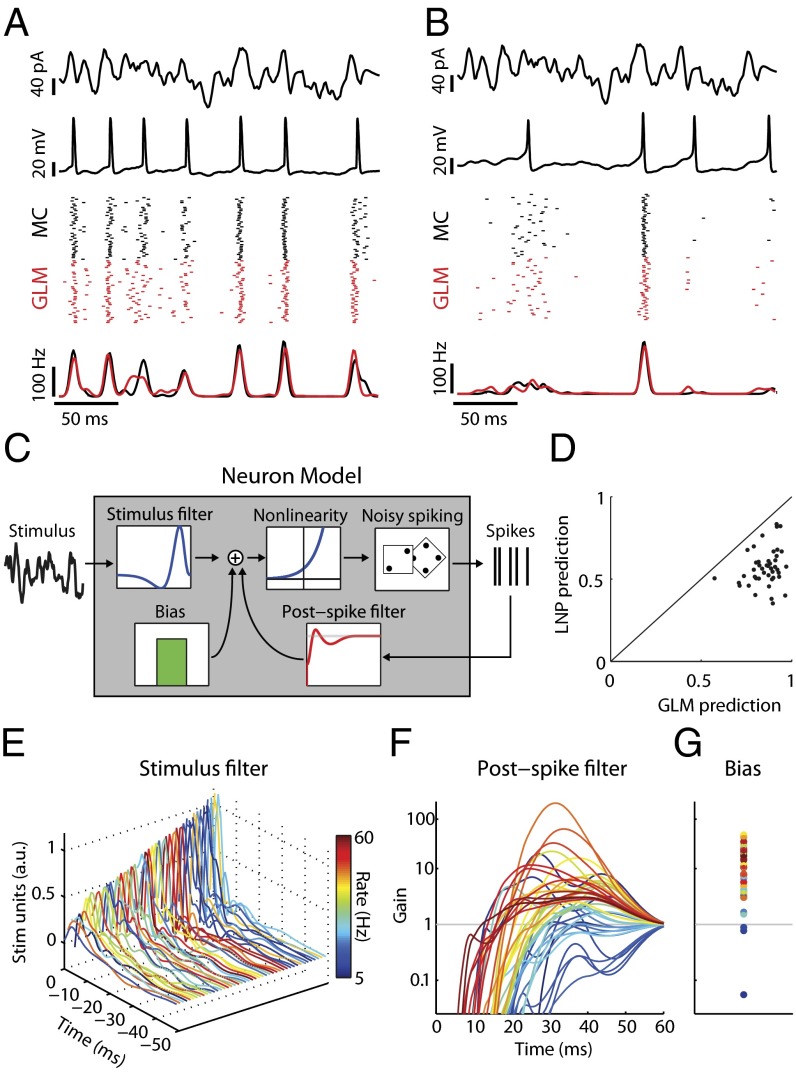
Simple models capture mitral cell stimulus-evoked responses and intrinsic diversity. (A) MC intrinsic properties are probed using filtered broadband stimuli (first row) injected somatically to evoke changes in membrane voltage (second row). Spike rasters (third row; black) and peri-stimulus time histograms (PSTH) (fourth row; black) for repeated stimulus presentations (n = 40 trials) show that this MC spikes to the stimulus with temporal jitter and displays a coarse stimulus preference. Model neuron rasters (Third Row, red) and PSTH (fourth row, red) show that the model accurately predicts MC activity on novel stimuli. (B) Same as A but for a different neuron. (C) Structure of the GLM neuron model. Model parameters describe a temporal stimulus filter, a postspike filter, and a constant bias term. An exponential nonlinearity defines an instantaneous spike rate and is used to draw noisy spikes. (D) GLM models accurately predict 86 ± 11% (mean ± SEM) of stimulus-evoked activity across all MCs, computed as the correlation coefficient between MC and model PSTH. For all neurons, the GLM fits were better than LNP models. (E–G) Model parameters for all MCs. Each line corresponds to parameters for a unique neuron and is colored by mean firing rate. (E) Temporal stimulus filters model differential stimulus specificity of neurons. (F) Exponentiated postspike filters, plotted as a multiplicative gain in spike probability following a spike at t = 0 ms. Values less (greater) than 1 indicate a decreased (increased) spike probability. (G) Bias terms also show considerable variation. Same y axis as F.