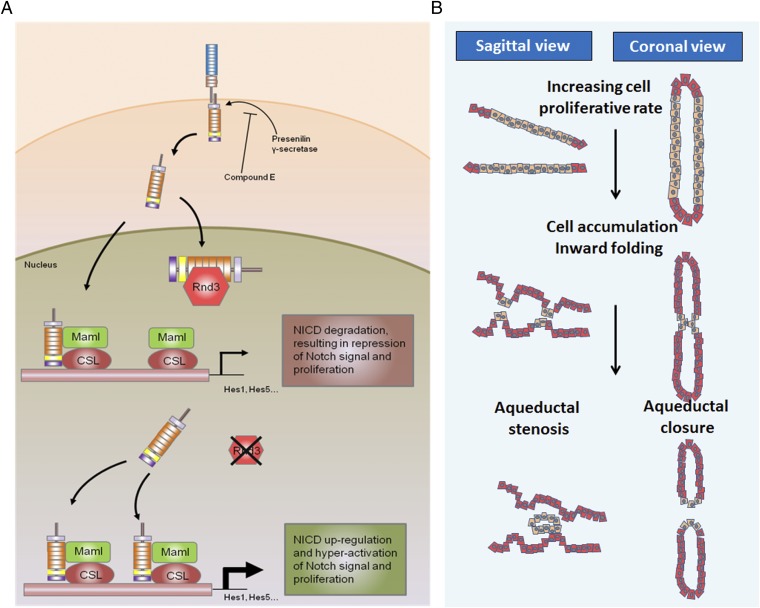
Fig. 8.
Proposed model outlining the molecular mechanism of the Rnd3 deficiency-mediated development of hydrocephalus. (A) On activation, Notch receptors on ependymal cell membranes are cleaved into the intracellular isoform NICD, which is translocated into the nucleus. In the nucleus, Rnd3 controls the accessibility of NICD protein to Maml and CSL by physically interacting with NICD. In the absence of Rnd3, Notch signaling is significantly enhanced owing to the extra amount of NICD available for Maml and CSL to form transcriptomes, which then facilitates ependymal cell proliferation, resulting in aqueduct stenosis and hydrocephalus. (B) The process of aqueduct stenosis formation. Depletion of Rnd3 promotes ependymal cell proliferation through the enhanced Notch signaling mechanism. The overgrowth of ependymal cells leads to the formation of multiple ependymal cell layers, inward cellular folding, and eventually luminal stenosis or closure.