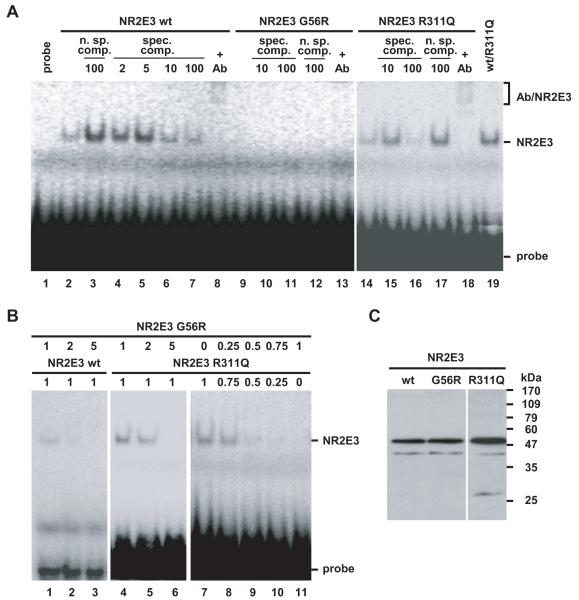
Figure 4.
The adRP-linked mutation p.G56R results in a dominant negative NR2E3 mutant protein. A) The p.G56R mutant protein is defective in DNA-binding. Binding of NR2E3 wild-type and mutant proteins to a radiolabeled response element was tested in EMSA by nonspecific (n. sp.) and specific (spec.) competition (comp.). Fold excess of cold probe is indicated. A specific anti-NR2E3 antibody (Ab) recognizes the NR2E3 dimer, inducing the formation of a slower migrating complex (‘supershift’ Ab/NR2E3). Contrast for lanes 1 to 13 was increased to show absence of binding of the p.G56R mutant protein and presence of the ‘supershift’ in lane 8. B) Dominant negative activity of p.G56R towards NR2E3 wild-type and p.R311Q proteins. Amounts of reticulocyte lysates are indicated in μl. C) Western Blot with anti-NR2E3 antisera on 10 μl of in vitro translated proteins. A major band at the expected size of 48 kDa for His-tagged NR2E3 is detected. The higher translation efficiency of pcDNA3.1-NR2E3R311Q contributes to a stronger signal detected by EMSA.