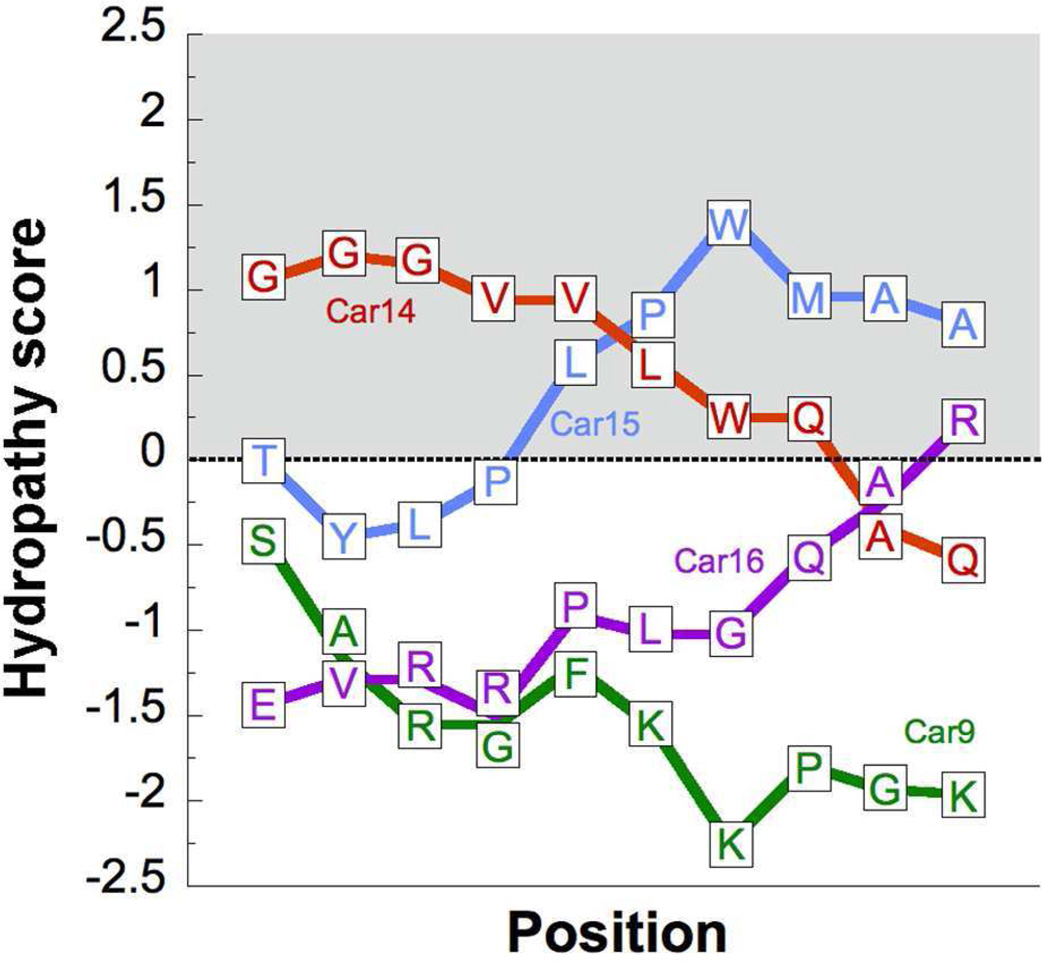
Figure 8.
Positional dependency of the hydrophobicity in carbon-binding peptides. The local hydrophobicity of the indicated Car sequences flanked by invariant tripeptides (Table 1) was determined with the ExPASy ProtScale tool (http://web.expasy.org/protscale/) using the Kyte and Doolittle hydrophobicity scale53 and a sliding window of 9 residues (thus, the first calculated hydropathy score is for the second residue of a Car dodecapeptide). Positive scores denote hydrophobic character.